Changes in Buprenorphine Treatment After Medicaid Expansion
Abstract
Objective:
The authors examined changes in buprenorphine treatment following Medicaid expansion, including the contribution of Medicaid-financed prescriptions.
Methods:
Buprenorphine pharmacy claims for patients were identified in the 2012–2018 IQVIA Longitudinal Prescription Data (LRx) data set, including 79.8% of U.S. retail prescriptions in 2012, increasing to 92.0% in 2018. A cohort analysis was used to assess the mean number of patients in a yearly quarter filling one or more buprenorphine prescriptions during preexpansion (2012–2013) and postexpansion (2014–2018) periods in expansion and nonexpansion states. Interrupted time-series analysis estimated associations of Medicaid expansion period with change in Medicaid-financed treatment. Separate analyses evaluated changes in duration and dose of new treatment episodes focused on mean quarterly number of patients treated with buprenorphine and proportions of new treatment episodes ≥180 days long and with ≥16 mg/day.
Results:
Between preexpansion and postexpansion, the mean quarterly number of patients taking buprenorphine increased by 93,300 in expansion states and by 84,960 in nonexpansion states. Corresponding changes for Medicaid-financed patients were 28,760 and 4,050, respectively. The fastest growth in Medicaid-financed treatment occurred among patients ages 25–44. Among new Medicaid-financed treatment episodes, little change was found in the proportion reaching the 180-day threshold, and declines were observed in the proportion receiving ≥16 mg/day.
Conclusions:
The findings are consistent with previous research indicating that Medicaid expansion has increased Medicaid-financed buprenorphine treatment. However, because of offsetting changes in other payment groups, the overall increase in expansion states was similar to the increase in nonexpansion states.
HIGHLIGHTS
Medicaid expansion was associated with a significant increase in Medicaid-financed patients who were treated with buprenorphine.
Because of coincident changes in other payment groups, this increase did not translate to greater overall increases in patients who were treated with buprenorphine in expansion compared with nonexpansion states.
Increases in buprenorphine treatment were largest among young adults, a group that has borne a particularly heavy burden of the opioid epidemic.
The United States is in the midst of a widespread opioid epidemic with far-reaching health consequences. Over the past decade, the number of U.S. adults who use heroin or misuse prescription opioids has increased, as have opioid-overdose rates (1, 2). Yet, few people with opioid use disorder receive substance use treatment (3).
Difficulty accessing affordable treatment is a leading reason for undertreatment of substance use disorders (4). Lack of health coverage can pose financial barriers to treatment, especially for adults with low income (5), who have elevated risks of substance use disorders (6). To help meet the health care needs of low-income adults, the Medicaid expansion provision of the Patient Protection and Affordable Care Act of 2010 (ACA) was implemented in January 2014. Although expansion increased Medicaid coverage to approximately 13.1 million low-income Americans (7), several states opted out of this provision. This decision may have had important implications for access to substance use treatment (8).
Buprenorphine treatment can effectively manage opioid withdrawal and prevent relapse, and buprenorphine is the most widely prescribed medication for opioid use disorder (9–13). After expansion, Medicaid-financed buprenorphine prescriptions increased faster in expansion than in nonexpansion states (14, 15). However, it is unknown whether these gains translated into population-wide differences in buprenorphine treatment across expansion and nonexpansion states or whether the policy resulted in improved treatment retention and dosing of buprenorphine. In this study, we provide the first population-wide assessment of the effects of Medicaid expansion on buprenorphine treatment patterns and on the quality of buprenorphine treatment among patients receiving Medicaid.
Recent ecological research (16–18), which has received extensive attention in the media (19, 20), has linked Medicaid expansion to slower growth in opioid overdose (16), drug overdose (17), and substance use disorder–related deaths in expansion states (18). It has been hypothesized that Medicaid expansion reduced overdose deaths by differentially increasing buprenorphine treatment in expansion states (16, 18). In support of this hypothesis, opioid overdose accounts for most drug overdose deaths (21), buprenorphine is the most widely prescribed medication for opioid use disorder (9), and consistent buprenorphine treatment lowers the risk for death among patients with opioid use disorder (22). Yet, most adults with opioid use disorder have private insurance (23), and the effects of Medicaid expansion on buprenorphine treatment at a national population level have not been previously examined.
To evaluate whether Medicaid expansion had population-wide effects on buprenorphine treatment consistent with greater declines in opioid-related deaths in expansion states compared with nonexpansion states, we characterized national changes in buprenorphine treatment after Medicaid expansion. We hypothesized that Medicaid expansion would drive greater population-wide increases in buprenorphine-treated patients in expansion states than in nonexpansion states. We also explored whether expansion spurred changes in the fraction of Medicaid-financed patients reaching benchmarks in buprenorphine treatment retention and dosing quality.
Methods
Data Sources
The analysis was based on all buprenorphine prescriptions to patients ages 16–80 years with an indication for opioid use disorder in the IQVIA Longitudinal Prescription Data (LRx) data set from January 2012 to December 2018. IQVIA LRx is a longitudinal database of prescriptions from retail pharmacies for individuals followed up across years, pharmacies, and payment sources. The estimated proportion of U.S. retail prescriptions covered in the data set increased from 79.8% in 2012 to 92.0% in 2018. In one analysis, we used publicly available state-level data on past-year nonmedical opioid use from the National Survey on Drug Use and Health (NSDUH) (24), a nationally representative civilian noninstitutionalized population survey, to estimate rates of buprenorphine treatment per 100 individuals who misused opioids, that is, used them nonmedically. In the NSDUH, opioid misuse was defined as use “in any way a doctor did not direct you to use it.” The deidentified data were exempted from review by the institutional review board of Yale University.
Buprenorphine and Demographic Groups
Prescriptions including buprenorphine were identified in IQVIA LRx. Buprenorphine formulations not approved for opioid use disorder were excluded from the analysis. We classified patients receiving buprenorphine by sex and age group and hierarchically by payment source (private insurance, Medicaid, other public insurance, or self-pay).
Expansion States and Study Periods
On the basis of the Kaiser Family Foundation legislative review, states were partitioned by implementation of the Medicaid State Plan Amendment provision (25). By the end of 2014, 26 states and the District of Columbia had implemented the Medicaid expansion and are referred to as expansion states. The remaining 24 states are referred to as nonexpansion states (see online supplement to this article).
Dose and Duration of New Buprenorphine Treatment Episodes
Some analyses were restricted to new buprenorphine treatment episodes. New episodes started on the date of a buprenorphine fill, with no fill in the previous 180 days, and ended after >30 days without another buprenorphine supply (13). New buprenorphine episodes were examined with respect to the percentage of episodes that were ≥180 days long and that included one or more buprenorphine prescriptions with a dose of ≥16 mg/day. These definitions followed the National Quality Forum measure that recommends ≥180 days of buprenorphine treatment (26), reports that higher daily buprenorphine doses predict better treatment retention (27, 28), and guideline targets of 16 mg/day (29).
Analysis
The primary analyses were conducted in four stages. First, the mean number of patients in a yearly quarter who had one or more buprenorphine prescriptions filled during the preexpansion (2012–2013) and postexpansion (2014–2018) periods was compared in expansion and nonexpansion states overall and by age, sex, and insurance coverage groups. Temporal trends were also plotted for the total number of patients filling buprenorphine prescriptions in expansion and nonexpansion states. The results accounted for changes in IQVIA LRx national coverage of U.S. retail prescriptions (79.8% in 2012 with a gradual increase to 92.0% in 2018).
Second, we sought to control total buprenorphine treatment patterns in expansion and nonexpansion states for population-based clinical need. We compared the rates of patients prescribed buprenorphine in expansion and nonexpansion states per 100 persons with past-year nonmedical prescription opioid use in each year derived from the NSDUH (24). Unlike some proxies for treatment need, such as opioid overdose, statewide nonmedical opioid use was likely to be relatively unaffected by buprenorphine-prescribing patterns.
Third, we used an interrupted time-series analysis to estimate the association of Medicaid expansion with a change among Medicaid-financed patients treated with buprenorphine and to assess whether this change differed between expansion and nonexpansion states. This quasi-experimental design controlled for secular quarterly trends and tested whether the policy change was associated with a change in the slope of buprenorphine treatment rates. We estimated changes in buprenorphine use associated with the policy using the difference between the counterfactual outcome based on the pretrend and the predicted outcome based on the posttrend. The changes corresponded to the difference in the number of Medicaid patients who were treated with buprenorphine had the policy not been enacted. Prais-Winsten regressions accounted for first-order autocorrelation. In separate models, we performed difference-in-difference estimates with state as a fixed effect. The p value associated with an interaction term of policy period with expansion state was used to estimate the strength of the differential effect of Medicaid expansion on trends in buprenorphine treatment in expansion and nonexpansion states. We also plotted change over time in the proportion of total patients being treated with buprenorphine covered by each payment source in expansion and nonexpansion states.
Fourth, we evaluated the effects of Medicaid expansion on the proportion of new buprenorphine treatment episodes that were ≥180 days long and on the proportion that included one or more prescriptions for ≥16 mg/day. Because IQVIA LRx data were available only through 2018, these analyses included episodes that started through the end of 2017. As a sensitivity analysis, we examined trends among all patients and among Medicaid-financed patients prescribed buprenorphine, excluding states that expanded Medicaid coverage before 2014 or between 2015 through 2018 (see online supplement).
Results
Trends Among All Patients Treated With Buprenorphine
Over the 2012–2018 period, roughly parallel increases occurred in the mean quarterly number of all patients treated with buprenorphine in expansion and nonexpansion states (Figure 1). Between the pre- and postexpansion periods, in each yearly quarter the number of patients treated with buprenorphine increased by 93,300 (95% confidence interval [CI]=59,570 to 127,020) in expansion states and by 84,960 (95% CI=55,590 to 114,320) in nonexpansion states (Table 1), a difference that was not statistically significant. In both expansion and nonexpansion states, the increases were largest among patients ages 25–44, and buprenorphine prescriptions significantly declined among people ages 16–24.
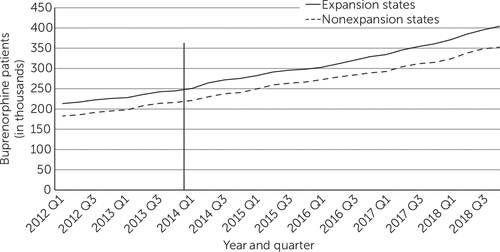
FIGURE 1. Mean quarterly number of patients treated with buprenorphine in expansion and nonexpansion states, 2012–2018a
aData source: 2012–2018 IQVIA Longitudinal Prescription Data. The vertical line shows when the Medicaid expansion took place.
| Variable | Medicaid expansion states | Medicaid nonexpansion states | ||||||
|---|---|---|---|---|---|---|---|---|
| Preexpansion (2012–2013) | Postexpansion (2014–2018) | Difference | 95% CI | Preexpansion (2012–2013) | Postexpansion (2014–2018) | Difference | 95% CI | |
| All | 228.85 | 322.15 | 93.30 | 59.57 to 127.02 | 198.99 | 283.94 | 84.96 | 55.59 to 114.32 |
| Age in years | ||||||||
| 16–24 | 28.78 | 20.67 | −8.11 | −11.20 to −5.02 | 22.56 | 17.71 | −4.85 | −7.39 to −2.31 |
| 25–34 | 86.61 | 115.24 | 28.63 | 20.49 to 36.76 | 80.27 | 105.39 | 25.13 | 18.61 to 31.64 |
| 35–44 | 53.32 | 91.00 | 37.68 | 23.46 to 51.89 | 49.01 | 85.30 | 36.29 | 23.00 to 49.57 |
| 45–54 | 36.89 | 53.31 | 16.42 | 10.15 to 22.69 | 29.26 | 43.51 | 14.25 | 8.35 to 20.15 |
| 55–80 | 23.25 | 41.93 | 18.68 | 10.94 to 26.42 | 17.88 | 32.03 | 14.14 | 8.15 to 20.13 |
| Sex | ||||||||
| Male | 139.07 | 191.88 | 52.82 | 34.48 to 71.15 | 118.41 | 165.16 | 46.75 | 31.01 to 62.49 |
| Female | 89.78 | 130.27 | 40.48 | 25.07 to 55.89 | 80.57 | 118.79 | 38.20 | 24.57 to 51.84 |
| Payment | ||||||||
| Medicaid | 28.23 | 57.00 | 28.76 | 15.66 to 41.87 | 23.10 | 27.15 | 4.05 | 1.25 to 6.84 |
| Private insurance | 178.29 | 234.02 | 55.74 | 38.70 to 72.77 | 154.26 | 220.73 | 66.47 | 45.29 to 87.66 |
| Other public insurance | 13.35 | 22.24 | 8.89 | 4.78 to 13.01 | 11.01 | 18.76 | 7.75 | 4.02 to 11.49 |
| Self-pay | 8.98 | 8.89 | −.10 | −.44 to .25 | 10.61 | 17.29 | 6.68 | 4.17 to 9.19 |
TABLE 1. Mean quarterly number of patients treated with buprenorphine in Medicaid expansion and nonexpansion states before and after expansiona
In expansion states, the mean quarterly number of treated patients covered by Medicaid increased by 28,760 (95% CI=15,660 to 41,870), whereas the corresponding increase in nonexpansion states was more modest (4,050 patients, 95% CI=1,250 to 6,840). As a result of the disproportionate increase in Medicaid-financed buprenorphine treatment in expansion states, the percentage of total Medicaid-paid buprenorphine prescriptions in expansion states nearly doubled from 9.87% (95% CI=9.80 to 9.94) to 18.91% (95% CI=18.85 to 18.97) during the study period, whereas the corresponding increase in nonexpansion states was smaller, from 7.53% (95% CI=7.47 to 7.59) to 7.90% (95% CI=7.86 to 7.95) (see online supplement).
Buprenorphine Treatment per 100 Persons With Nonmedical Opioid Use
During the study period, the rates of buprenorphine treatment per 100 persons with past-year nonmedical prescription opioid use were similar in expansion and nonexpansion states (Figure 2). Between 2012 and 2018, these rates increased from 5.5 to 10.9 per 100 persons in expansion states and from 5.7 to 10.5 in nonexpansion states.
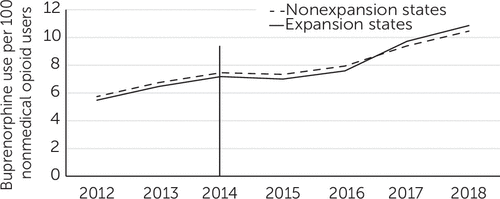
FIGURE 2. Rate of buprenorphine use per 100 persons with past-year nonmedical prescription opioid use in expansion and nonexpansion states, 2012–2018a
aData source: 2012–2018 IQVIA Longitudinal Prescription Data and National Survey on Drug Use and Health. The vertical line shows when the Medicaid expansion took place.
Trends Among Medicaid-Financed Patients Treated With Buprenorphine
During the preexpansion period, the numbers of and changes in Medicaid patients treated with buprenorphine in each yearly quarter were similar in expansion and nonexpansion states. After the policy intervention, however, the rise in buprenorphine treatment was greater in expansion than in nonexpansion states (Figure 3). During the postexpansion period, an estimated 59,830 Medicaid patients per quarter were treated with buprenorphine in expansion states who would not have been treated had the policy not been enacted. The corresponding number in nonexpansion states was 16,730 (see online supplement). The largest gains occurred among adults ages 25–34 in expansion states, followed by those ages 35–44 (see online supplement).
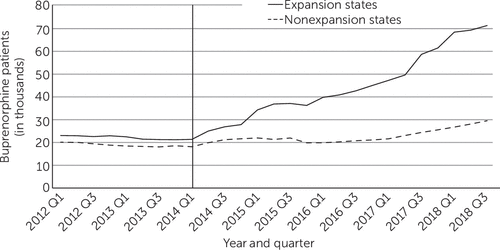
FIGURE 3. Mean quarterly number of Medicaid-financed patients treated with buprenorphine in expansion and nonexpansion states, 2012–2018a
aData source: 2012–2018 IQVIA Longitudinal Prescription Data. The vertical line shows when the Medicaid expansion took place.
After excluding from the analysis states that expanded Medicaid before 2014 or in 2015 through 2018, an estimated 43,850 (95% CI=17,990 to 69,220) additional Medicaid patients at the end of the expansion period received buprenorphine in expansion states, whereas the estimated policy-related increase was not statistically significant in nonexpansion states (9,260 patients, 95% CI=−1,030 to 19,560).
Among new episodes of buprenorphine treatment of Medicaid patients, no significant change occurred in the proportion reaching the 180-day duration threshold in expansion states (increase of 21.9% to 24.6%, difference=2.7 percentage points, 95% CI=−7.4 to 12.7) and nonexpansion states (23.1% to 28.0%, difference=4.9 percentage points, 95% CI=−5.6 to 15.5). A significant decline in the proportion that included prescriptions for ≥16 mg/day was detected in expansion states (from 68.2% to 62.7%, difference=−5.5 percentage points, 95% CI=−9.3 to 1.7) and a nearly statistically significant decline was observed in nonexpansion states (from 73.4% to 70.5%, difference=−2.9 percentage points, 95% CI=−5.9 to 0.0).
Trends Among Non–Medicaid-Financed Patients Treated With Buprenorphine
During this period, the mean quarterly number of self-pay patients who were treated with buprenorphine increased from 10,610 to 17,290 in nonexpansion states and declined slightly from 8,980 to 8,890 in expansion states (Table 1 and Figure 4). Trends among buprenorphine-treated patients who were financed through private insurance and other public insurance were similar in expansion and nonexpansion states (see online supplement).
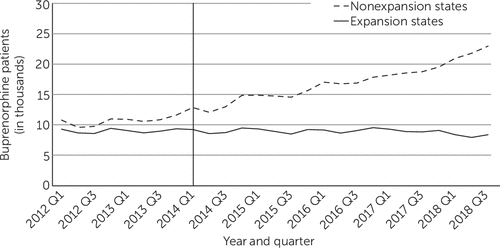
FIGURE 4. Mean quarterly number of self-pay patients treated with buprenorphine in expansion and nonexpansion states, 2012–2018a
aData source: 2012–2018 IQVIA Longitudinal Prescription Data. The vertical line shows when the Medicaid expansion took place.
Discussion
Medicaid expansion coincided with a disproportionate increase in the number of Medicaid-financed patients receiving buprenorphine in expansion states. This increase did not, however, translate into a significantly greater population-wide increase in buprenorphine treatment in expansion states, compared with nonexpansion states. Because of offsetting increases among buprenorphine-treated patients who were uninsured or who had other payment sources in nonexpansion states, we observed nearly parallel increases in the total number of buprenorphine-treated patients in expansion and nonexpansion states. Similar trends were observed in buprenorphine treatment rates of people who misused prescription opioids. Despite these increases, the national volume of buprenorphine treatment remained remarkably low, estimated at approximately 300,000 per yearly quarter, far below a conservative (30) estimate of approximately two million U.S. individuals with opioid use disorder (31).
Although Medicaid is a vital source of health care coverage for people with low income and disabilities, it accounts for only a modest proportion of overall buprenorphine treatment coverage in the United States. During the years immediately before expansion, Medicaid covered 12% of buprenorphine treatment episodes in expansion and nonexpansion states. Given its modest coverage contribution to the total national buprenorphine supply, it is not surprising that the increase in Medicaid-covered buprenorphine treatment in expansion states did not drive a significant overall increase in buprenorphine treatment in these states. The increase in Medicaid-financed treatment in expansion states was offset by increases in self-pay in nonexpansion states. Left without insurance, those in need of treatment in nonexpansion states may have opted to pay out of pocket. This pattern suggests that one important function of Medicaid coverage was to reduce financial burden arising from out-of-pocket costs.
Private insurance was the dominant buprenorphine payment source during the study period, accounting for more than three-quarters of patients who were treated with buprenorphine. This percentage exceeds the estimated 53% of people with opioid use disorder who have private insurance (23). Our findings suggest that privately insured individuals with treatment needs were disproportionately treated with buprenorphine. This finding is consistent with previous work indicating that buprenorphine tends to be provided in more affluent and less racially diverse communities (32, 33) and that substance use clinics that offer medications for opioid use disorder tend to accept private insurance (34).
The treatment trends we report here have implications for recent ecological research reporting that Medicaid expansion has slowed substance use–related mortality (16–18). These findings have led to the hypothesis that mortality reductions in expansion states are due to increased buprenorphine treatment (16, 18). However, population-wide buprenorphine treatment trends cast doubt on whether Medicaid-financed gains in buprenorphine treatment have slowed overall drug-related mortality rates more in expansion states than in nonexpansion states. Nevertheless, it is conceivable that greater buprenorphine-related reductions in mortality rates in expansion states were associated with the outsized impact on Medicaid patients, who may have higher illness severity (35) and mortality risk than other patients who are treated with buprenorphine. It is also possible that other factors may have accounted for these reductions in mortality rates, such as Medicaid-related improvement in financial security (36), Medicaid-financed naloxone-based overdose reversal (37), methadone or naltrexone treatment, and other Medicaid-financed mental health or substance services (14).
Our findings extend previous research on the volume of buprenorphine prescribing after Medicaid expansion (14, 15) by including national trends in the number of patients prescribed buprenorphine. Consistent with the previous research, we noted that Medicaid expansion was associated with an increase in Medicaid-financed buprenorphine treatment that was greater in expansion than in nonexpansion states. Some previous studies, including a natural coverage experiment, the Oregon Medicaid lottery (38), and a time-series study based on population (39), did not find a significant increase in opioid use disorder treatment after Medicaid coverage. However, these studies were likely underpowered to evaluate this outcome.
Our results contrast with a five-state study of 2011–2016 IQVIA LRx data that reported an increase in the overall rate of nonelderly adults treated with buprenorphine in counties of expansion states, compared with nonexpansion states (40). In that analysis, significant corresponding changes were not found in Medicaid-financed buprenorphine prescriptions or number of medication days. Differences in study populations, study periods, units of analysis, and analytical methods may help to explain these contrasting results.
Several factors may have contributed to overall rising rates of buprenorphine treatment in expansion and nonexpansion states during the study period. First, the burden of opioid use disorder increased during this period. Second, practice guidelines and clinical research published during that time supported buprenorphine’s effectiveness for managing opioid use disorder. Third, federal policies increased buprenorphine prescriber waiver limits and broadened the range of health care specialists eligible to receive waivers. Finally, several other contemporaneous health care reforms may have played a role. These reforms included Marketplace exchanges that enrolled >10 million people in private health insurance each year from 2016 to 2018 (41), most of which provided at least some coverage of buprenorphine; the Drug Addiction Treatment Act of 2016, which increased buprenorphine patient limits for individual prescribers from 100 to 275; and the Comprehensive Addiction and Recovery Act of 2016, which extended buprenorphine waiver options to physician assistants and nurse practitioners.
Adults ages 25–44, have borne a particularly heavy burden of the opioid epidemic, with high and rapidly increasing rates of opioid-involved overdose deaths (42). It was therefore encouraging that the fastest growth of Medicaid-financed buprenorphine treatment occurred in this age group. It was also encouraging that the rate of buprenorphine treatment of those who misused prescription opioids increased during the study period.
Problems with retaining patients on buprenorphine (43) and achieving adequate dosing undermine the effectiveness of buprenorphine treatment in community practice (44). In addition to increasing treatment access through Medicaid expansion, the ACA offered hope of improving the quality of substance use treatment by integrating and coordinating the care of dually eligible Medicaid and Medicare beneficiaries with substance use disorders via Medicaid health homes and coordinated care organizations (45). The present analysis, however, provides no evidence of improvements over time in the proportion of Medicaid-covered, buprenorphine-treated patients reaching dosing and duration thresholds. Instead, we observed a nonsignificant trend toward a declining proportion of new episodes reaching the dosing threshold, which raises concerns about a decrease in quality of buprenorphine treatment during a period of rapid growth in buprenorphine prescribing that may be related to less-experienced buprenorphine prescribers (46) or to changes in patient case mix.
This analysis had some limitations. First, the accuracy of population IQVIA coverage estimates was uncertain. Second, the prescription data represented buprenorphine purchases rather than consumption, and assessment of illicit diversion was not possible (47). Third, we could not exclude patients treated with off-label buprenorphine prescribing for chronic pain. This group accounts for approximately 11% of buprenorphine prescriptions (48). Finally, the NSDUH survey item concerning nonmedical prescription opioid use is a crude and broad proxy to index trends in treatment need.
Conclusions
Although Medicaid expansion succeeded in increasing the number of Medicaid-covered patients who were treated with buprenorphine, population-wide effects were offset by changes in other patient payment groups. Within expansion states, the increase among Medicaid patients treated with buprenorphine was concentrated among younger adults, the age group at highest risk for opioid-related overdose deaths. Although progress has been made in extending buprenorphine access in expansion and nonexpansion states, widespread challenges exist in further increasing buprenorphine treatment access, improving treatment retention, and reducing the financial burden of uninsured people, especially in nonexpansion states. To meet community treatment needs, access to buprenorphine or other effective medications should be expanded in established treatment settings, such as substance use clinics, office-based practice, and federally qualified health centers, as well as the publicly funded mental health system.
1 : Vital signs: trends in emergency department visits for suspected opioid overdoses—United States, July 2016–September 2017 . MMWR Morb Mortal Wkly Rep 2018 ; 67 : 279 – 285 Crossref, Medline, Google Scholar
2 : Trends and key correlates of prescription opioid injection misuse in the United States . Addict Behav 2018 ; 78 : 145 – 152 Crossref, Medline, Google Scholar
3 : Trends in Substance Use Disorders Among Adults Aged 18 or Older. Rockville, MD , Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2017 Google Scholar
4 : Reasons for not seeking substance use disorder treatment: variations by health insurance coverage . J Behav Health Serv Res 2017 ; 44 : 63 – 74 Crossref, Medline, Google Scholar
5 Behavioral Health Trends in the United States: Results From the 2014 National Survey on Drug Use and Health. Pub no SMA-15-4927. Rockville, MD, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2015Google Scholar
6 : The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) waves 1 and 2: review and summary of findings . Soc Psychiatry Psychiatr Epidemiol 2015 ; 50 : 1609 – 1640 Crossref, Medline, Google Scholar
7 Medicaid Enrollment Changes Following the ACA. Washington, DC, Medicaid and CHIP Payment and Access Commission, 2020. https://www.macpac.gov/subtopic/medicaid-enrollment-changes-following-the-aca. Accessed Oct 13, 2020Google Scholar
8 : The Affordable Care Act and opioid agonist therapy for opioid use disorder . Psychiatr Serv 2019 ; 70 : 617 – 620 Link, Google Scholar
9 : National and state treatment need and capacity for opioid agonist medication-assisted treatment . Am J Public Health 2015 ; 105 : e55 – e63 Crossref, Medline, Google Scholar
10 Medication-Assisted Treatment for Substance Use Disorders. Baltimore , Centers for Medicare and Medicaid Services , 2014 . http://medicaid.gov/Federal-Policy-Guidance/Downloads/CIB-07-11-2014.pdf Google Scholar
11 : Medication-assisted therapies—tackling the opioid-overdose epidemic . N Engl J Med 2014 ; 370 : 2063 – 2066 Crossref, Medline, Google Scholar
12 : Moving addiction care to the mainstream—improving the quality of buprenorphine treatment . N Engl J Med 2018 ; 379 : 4 – 6 Crossref, Medline, Google Scholar
13 : Buprenorphine treatment by primary care providers, psychiatrists, addiction specialists, and others . Health Aff 2020 ; 39 : 984 – 992 Crossref, Google Scholar
14 : Impact of Medicaid expansion on access to opioid analgesic medications and medication-assisted treatment . Am J Public Health 2018 ; 108 : 642 – 648 Crossref, Medline, Google Scholar
15 : Impact of Medicaid expansion on Medicaid-covered utilization of buprenorphine for opioid use disorder treatment . Med Care 2017 ; 55 : 336 – 341 Crossref, Medline, Google Scholar
16 : Association of Medicaid expansion with opioid overdose mortality in the United States . JAMA Netw Open 2020 ; 3 :
17 : Early Medicaid expansions and drug overdose mortality in the USA: a quasi-experimental analysis . J Gen Intern Med 2019 ; 34 : 23 – 25 Crossref, Medline, Google Scholar
18 : Association between state Medicaid eligibility thresholds and deaths due to substance use disorders . JAMA Netw Open 2019 ; 2 :
19 : Obamacare May Have Prevented Many Opioid-Related Deaths. Norwalk, CT , HealthDay , 2020 . https://www.usnews.com/news/health-news/articles/2020-01-10/obamacare-may-have-prevented-many-opioid-related-deaths . Accessed Oct 12, 2020 Google Scholar
20 Bernstein L: Medicaid Expansion May Have Saved Thousands From Drug Overdose Deaths. Washington, DC, Washington Post, 2020. https://www.washingtonpost.com/health/medicaid-expansion-may-have-saved-thousands-from-drug-overdose-deaths/2020/01/10/08cfe828-3334-11ea-a053-dc6d944ba776_story.html. Accessed Oct 13, 2020Google Scholar
21 Drug Overdose Deaths. Atlanta, Centers for Disease Control and Prevention, 2020. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed June 21, 2020Google Scholar
22 : Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies . BMJ 2017 ; 357 : j1550 Crossref, Medline, Google Scholar
23 : Opioid use disorder in the United States: insurance status and treatment access . Drug Alcohol Depend 2008 ; 94 : 207 – 213 Crossref, Medline, Google Scholar
24 2017–2018 NSDUH Estimated Totals by State. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2020. https://www.samhsa.gov/data/report/2017-2018-nsduh-estimated-totals-state. Accessed June 20, 2020Google Scholar
25 Status of State Action on the Medicaid Expansion Decision. Menlo Park, CA, Kaiser Family Foundation, 2020. https://www.kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed Oct 13, 2020Google Scholar
26 Behavioral Health 2016–2017 Final Report. Washington, DC, National Quality Forum, 2017. https://www.qualityforum.org/Publications/2017/08/Behavioral_Health_2016-2017_Final_Report.aspx. Accessed Oct 13, 2020Google Scholar
27 : Analysis of buprenorphine/naloxone dosing impact on treatment duration, resource use and costs in the treatment of opioid-dependent adults: a retrospective study of US public and private health care claims . Postgrad Med 2014 ; 126 : 113 – 120 Crossref, Medline, Google Scholar
28 : Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial . Addiction 2014 ; 109 : 79 – 87 Crossref, Medline, Google Scholar
29 Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, MD , Substance Abuse and Mental Health Services Administration , 2004 Google Scholar
30 : Estimated prevalence of opioid use disorder in Massachusetts, 2011–2015: a capture-recapture analysis . Am J Public Health 2018 ; 108 : 1675 – 1681 Crossref, Medline, Google Scholar
31 2018 NSDUH Detailed Tables. Rockville, MD, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2019. https://www.samhsa.gov/data/report/2018-nsduh-detailed-tables. Accessed Oct 13, 2020Google Scholar
32 : Variation in use of buprenorphine and methadone treatment by racial, ethnic, and income characteristics of residential social areas in New York City . J Behav Health Serv Res 2013 ; 40 : 367 – 377 Crossref, Medline, Google Scholar
33 : Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States . JAMA Netw Open 2020 ; 3 :
34 : Medication treatment for opioid use disorders in substance use treatment facilities . Health Aff 2019 ; 38 : 14 – 23 Crossref, Medline, Google Scholar
35 : Characteristics and treatment patterns of US commercially insured and Medicaid patients with opioid dependence or abuse . J Opioid Manag 2017 ; 13 : 207 – 220 Crossref, Medline, Google Scholar
36 : Health insurance coverage and health—what the recent evidence tells us . N Engl J Med 2017 ; 377 : 586 – 593 Crossref, Medline, Google Scholar
37 : The impact of expanded Medicaid eligibility on access to naloxone . Addiction 2019 ; 114 : 1567 – 1574 Crossref, Medline, Google Scholar
38 : The effect of Medicaid on medication use among poor adults: evidence from Oregon . Health Aff 2017 ; 36 : 2110 – 2114 Crossref, Google Scholar
39 : Trends in insurance coverage and treatment among persons with opioid use disorders following the Affordable Care Act . Drug Alcohol Depend 2017 ; 179 : 271 – 274 Crossref, Medline, Google Scholar
40 : Changes in buprenorphine-naloxone and opioid pain reliever prescriptions after the Affordable Care Act Medicaid expansion . JAMA Netw Open 2018 ; 2 :
41 Health Insurance Exchanges 2018 Open Enrollment Period Final Report. Baltimore, Centers for Medicare and Medicaid Services, 2018. https://www.cms.gov/newsroom/fact-sheets/health-insurance-exchanges-2018-open-enrollment-period-final-report. Accessed Oct 13, 2020Google Scholar
42 : Racial/ethnic and age group differences in opioid and synthetic opioid-involved overdose deaths among adults aged ≥18 years in metropolitan areas—United States, 2015–2017 . MMWR Morb Mortal Wkly Rep 2019 ; 68 : 967 – 973 Crossref, Medline, Google Scholar
43 : Retention in medication-assisted treatment for opiate dependence: a systematic review . J Addict Dis 2016 ; 35 : 22 – 35 Crossref, Medline, Google Scholar
44 : Patterns of buprenorphine-naloxone treatment for opioid use disorder in a multistate population . Med Care 2017 ; 55 : 669 – 676 Crossref, Medline, Google Scholar
45 : The Affordable Care Act will revolutionize care for substance use disorders in the United States . Addiction 2014 ; 109 : 1957 – 1958 Crossref, Medline, Google Scholar
46 : Patterns and quality of buprenorphine opioid agonist treatment in a large Medicaid program . J Addict Med 2015 ; 9 : 470 – 477 Crossref, Medline, Google Scholar
47 : Diversion of buprenorphine/naloxone coformulated tablets in a region with high prescribing prevalence . J Addict Dis 2009 ; 28 : 226 – 231 Crossref, Medline, Google Scholar
48 : Using a learning collaborative strategy with office-based practices to increase access and improve quality of care for patients with opioid use disorders . J Addict Med 2016 ; 10 : 117 – 123 Crossref, Medline, Google Scholar



