Cost-Effectiveness of a Web-Based Program for Residual Depressive Symptoms: Mindful Mood Balance
Abstract
Objective:
Mindful Mood Balance (MMB) is an effective Web-based program for residual depressive symptoms that prevents relapse among patients with partial recovery from major depressive episodes. This cost-effectiveness analysis was conducted from the health plan perspective alongside a pragmatic randomized controlled trial of MMB.
Methods:
Adults were recruited from behavioral health and primary care settings in a large integrated health system and randomly assigned to MMB plus usual depression care (MMB+UDC) or UDC. Patients had at least one prior major depressive episode; a current score of 5–9 on the Patient Health Questionnaire–9, indicating residual depressive symptoms; and Internet access. Program costs included recruitment, coaching, and MMB licensing. Center for Medicare and Medicaid fee schedules were applied to electronic health record utilization data for psychotropic medications and psychiatric and psychotherapy visits. Effectiveness was measured as depression-free days (DFDs), converted from PHQ-9 scores collected monthly for 1 year. Incremental cost-effectiveness ratios were calculated with various sets of cost inputs.
Results:
A total of 389 patients (UDC, N=210; MMB+UDC, N=179) had adequate follow-up PHQ-9 measures for inclusion. MMB+UDC patients had 29 more DFDs during follow-up. Overall, the incremental cost of MMB+UDC was $431.54 over 12 months. Incremental costs per DFD gained ranged from $9.63 for program costs only to $15.04 when psychiatric visits, psychotherapy visits, and psychotropic medications were included.
Conclusions:
MMB offers a cost-effective Web-based program for reducing residual depressive symptoms and preventing relapse. Health systems should consider adopting MMB as adjunctive to traditional mental health care services.
Highlights
Mindful Mood Balance is a Web-based program that is closely aligned with mindfulness-based cognitive therapy and has been shown to reduce residual depressive symptoms and prevent major depressive relapse.
Over 12 months, patients randomly assigned to Mindful Mood Balance experienced 29 more depression-free days, compared with those assigned to usual depression care, at a cost of $10–$15 per depression-free day and an incremental 12-month cost of $432.
Mindful Mood Balance offers a cost-effective Web-based program for reducing residual depressive symptoms and preventing major depressive relapse that has costs comparable to or lower than other Web-based depression programs.
Major depression is a highly prevalent chronic condition, with high societal cost, and was the leading cause of disability in 2017 (1). Depression is associated with low educational and economic attainment; it also is associated with marital disruption and suicide, each of which incurs costs from lost productivity and health and mental health treatment (2–4). Residual depressive symptoms, which occur when patients partially recover from a major depressive episode, represent a pervasive ongoing burden that is often left untreated and increases the risk of major depressive relapse (5, 6).
Mindfulness-based cognitive therapy (MBCT) is an empirically supported program that teaches emotion regulation skills for reducing residual depressive symptoms and confers a 43% reduction in relapse risk (7–10). Like many psychotherapies, MBCT faces dissemination challenges, including shortages of MBCT-trained therapists, service cost, and logistical hurdles (11). These access barriers provided the rationale for a Web-based program informed by MBCT, called Mindful Mood Balance (MMB). MMB was evaluated in a large randomized controlled trial (N=460) and found effective for reducing residual depressive symptoms over a 12-month follow-up period (12). These data suggest that MMB may be a scalable solution for expanding availability of MBCT.
Results of the few cost-effectiveness analyses of Web-based depression treatments have been mixed. Some have shown cost-effectiveness within $20,000 or $50,000 per year willingness-to-pay thresholds (which are likely too high for U.S. health plans to consider), but none have shown significant reductions in costs from averted health care utilization (13–16). Internet-delivered cognitive-behavioral therapy programs average 50% probability for cost-effectiveness at a $0 willingness-to-pay threshold (17). Web-based programs may promote help-seeking behavior, leading to increased outpatient service utilization. Traditionally, this may be interpreted as a detrimental impact of Web-based programs from a cost-effectiveness standpoint, but the context of resource and access limitations within the U.S. mental health care system must also be considered.
Cost-effectiveness analyses from the health plan perspective, which directly examine actual costs associated with implementing the program as well as changes in health care service utilization attributable to the program, are particularly important for health plan decision makers who must consider the multitude of digital programs available. As the Centers for Medicare and Medicaid Services (CMS) explores the integration of behavioral health services into capitated systems, investing in effective, low-cost approaches to mental health care is particularly important (18).
This cost-effectiveness analysis of MMB from the health plan perspective was deployed inside a randomized controlled trial. The effectiveness outcomes of the randomized trial indicated significant reductions in both residual depressive symptoms and major depressive relapse (12). The hypothesis was that MMB would have a low cost per depression-free day (DFD) (a measure of depression severity used in longitudinal follow-up studies) within 1 year, relative to comparable programs that are typically available in health systems to treat patients to remission.
Methods
Participants
Participants were recruited from Kaiser Permanente Colorado (KPCO), an integrated health system serving approximately 630,000 patients from the Denver, Boulder, and surrounding metropolitan areas. Participants were randomly assigned to receive MMB and usual depression care (MMB+UDC; N=230) or UDC alone (N=230), with recruitment spanning from March 2, 2015, to November 30, 2018. Recruitment occurred through clinic flyers and provider referrals; however, the primary means was by administrative identification of potentially eligible patients with a depression diagnosis and a qualifying Patient Health Questionnaire–9 (PHQ-9) score during a routine visit. Patients were then sent an invitation e-mail for the trial that included a consent website (19). Inclusion criteria were minimal: ≥18 years of age (determined by medical record), at least one prior major depressive episode (medical record diagnosis of depression plus phone interview), current PHQ-9 score ≥5 and ≤9 (initially completed during a health care visit and confirmed via phone interview) (20), Internet access, and intention to continue KPCO membership for at least 6 months (phone interview). Exclusion criteria were determined by medical record review and included ICD-9 or ICD-10 diagnoses within the past 2 years of schizophrenia or current psychosis, organic mental disorder, or pervasive developmental delay.
Study Design
The cost-effectiveness of MMB+UDC versus UDC was determined. The study was conducted alongside a single-blind, two-group, randomized controlled trial that reported significant reductions in both depressive symptom severity and relapse of major depression (12). Briefly, through eight self-administered sessions, MMB uses vicarious learning (videos), experiential practice, and didactic information to teach mindfulness and meditation skills and increases awareness of dysfunctional automatic thought processes that contribute to depression.
The study was approved by the institutional review boards of Kaiser Permanente Colorado, University of Toronto, and University of Colorado Boulder. All patients provided informed consent and signed a HIPAA authorization for release of medical record information.
Effectiveness Measure
DFD is a standardized measure for cost-effectiveness studies of cumulative depression severity when there are multiple measurements over time. Patients were assessed for depression severity via monthly administration of a Web-based PHQ-9 for 12 months following the eight-session (12 weeks) MMB program. PHQ-9 scores were converted to DFDs by using a method that was previously developed (21). Because the first DFD score was constrained to a 5–9 range, a minimum of two measurement points (baseline and one follow-up) were required. Further, in a prior study, a single PHQ-9 value in a 12-month period was found to skew results substantially, compared with at least two values (21). Sensitivity analyses (not shown) were conducted to examine differences in DFD calculations between groups with different requirements for follow-up PHQ-9 completeness: one PHQ-9 score at any time, one initial and one late PHQ-9 score, and one initial plus three or more subsequent PHQ-9 scores. Differences of fewer than two DFDs were observed across these requirements, but more restrictive approaches excluded more patients, and thus the most inclusive approach was employed of requiring only one PHQ-9 score during follow-up (in addition to the baseline PHQ-9 measurement).
Cost Measures
Instruments previously employed for other behavioral health inventions (22, 23) were used to collect labor costs for recruitment and coaching (MMB only) via task- and time-tracking logs that were recorded by all study staff for 4 weeks at the start of the trial, 4 weeks in the middle, and 4 weeks at the end. Each participant received an average of 2.34 hours of coaching support. A description of coaching activities is provided elsewhere (12). Recruitment tasks included population-based outreach to patients via e-mail and enrollment website tracking. Tasks associated with eligibility screening, survey data collection, or other “research only” tasks were excluded. The mean hourly wage for a health education specialist as listed by the U.S. Bureau of Labor Statistics was used to estimate staff salaries (24). Overhead costs of 10% were applied for workspace and equipment (i.e., computers). Although patients were not charged for MMB as part of this research trial, MMB developers estimated a cost of $180 per patient in the future, which was included.
All ambulatory and acute health care services utilization data were extracted from the electronic health record (EHR) and claims databases. Medical costs were estimated with the standard relative resource cost algorithm (SRRCA) for all services provided and paid for by KPCO (25, 26). SRRCA applies CMS fee schedules to standardized claims or EHR-derived utilization data to ensure that observed differences are not a result of differing pricing methods and billing rules (bundling of services). These estimates do not, however, account for variation in patient cost-sharing or copays. Costs were divided into groups, including psychiatric and psychotherapy visits, psychotropic medication use, and emergency department visits and inpatient stays with primary or secondary psychiatric or substance abuse diagnoses; costs were inflation adjusted to 2015 U.S. dollars by using the medical care component of the Consumer Price Index (27, 28). Total and average costs were estimated during the 12-month follow-up period.
Statistical Analysis
Incremental cost-effectiveness ratios (ICERs) were calculated with DFDs as the effectiveness measure. Separate ICERs were calculated for different sets of cost inputs to highlight the impact of costs from different sources, because health system stakeholders may be more interested in specific cost impacts. Initially, ICERs considered only the labor costs, and then other costs were incrementally added for psychotherapy visits (mental health and substance abuse), antidepressant medications, and all psychotropic medications. The trial was not powered on cost outcomes but rather on the effectiveness measures for changes in depression symptoms; therefore, analysis of inpatient and emergency costs was exploratory, because this study was not powered to handle large cost fluctuations. Confidence intervals (95%) were constructed by using Fieller’s method (29, 30). A sensitivity analyses was conducted with all costs for the 230 patients, which added costs back in for those that were lost to follow-up.
Results
Among the 460 patients recruited for the trial, 389 (85%) had completed a PHQ-9 during the follow-up period and were included in the main analysis (UDC, N=210; MMB+UDC, N=179). Table 1 presents baseline demographic characteristics; no significant differences were noted on any self-reported demographic, risk factor, or treatment history variables. Patients in the MMB+UDC group had an average of 28.72 more DFDs in the 12-month follow-up period, compared with UDC (interquartile range=40.91).
| UDC | MMB+UDC | ||||
|---|---|---|---|---|---|
| (N=210) | (N=179) | ||||
| Characteristic | N | % | N | % | p |
| Female | 159 | 76 | 136 | 76 | .91 |
| Age (median, interquartile range) | 49 | 35–61 | 47 | 35–60 | .99 |
| Education | .78 | ||||
| No high school diploma | 3 | 1 | 2 | 1 | |
| High school diploma | 21 | 10 | 21 | 12 | |
| Bachelor’s degree | 85 | 41 | 64 | 36 | |
| Graduate-level degree | 99 | 48 | 90 | 51 | |
| Race | .95 | ||||
| Asian | 4 | 2 | 3 | 2 | |
| Black | 4 | 2 | 4 | 2 | |
| White | 187 | 90 | 163 | 92 | |
| American Indian, Alaska Native, Native Hawaiian, or other | 15 | 7 | 9 | 5 | |
| Hispanic ethnicity | 16 | 8 | 15 | 9 | .79 |
| Marital status | .63 | ||||
| Never married | 44 | 21 | 47 | 26 | |
| Married or domestic partnership | 105 | 50 | 77 | 43 | |
| Divorced | 47 | 23 | 44 | 25 | |
| Separated | 4 | 2 | 4 | 2 | |
| Widowed | 7 | 3 | 6 | 3 | |
| Annual income ($) | .94 | ||||
| 0–29,999 | 22 | 11 | 18 | 10 | |
| 30,000–69,999 | 81 | 39 | 70 | 40 | |
| 70,000–99,999 | 53 | 26 | 41 | 23 | |
| ≥100,000 | 51 | 25 | 47 | 27 | |
| Employment | .84 | ||||
| Disabled | 6 | 3 | 4 | 2 | |
| Student | 10 | 5 | 8 | 5 | |
| Full-time work | 108 | 51 | 97 | 54 | |
| Homemaker | 7 | 3 | 4 | 2 | |
| Other | 10 | 5 | 6 | 3 | |
| Part-time work | 28 | 13 | 16 | 9 | |
| Retired | 36 | 17 | 40 | 22 | |
| Unemployed | 5 | 2 | 4 | 2 | |
| Ever hospitalized for depression | 31 | 15 | 28 | 16 | .82 |
| Ever attempted suicide | 37 | 18 | 27 | 15 | .48 |
| Ever treated for drug or alcohol use | 18 | 9 | 11 | 6 | .37 |
| Frequency of marijuana use | .83 | ||||
| None | 159 | 76 | 139 | 79 | |
| Less than once per month | 20 | 10 | 11 | 6 | |
| Once or twice per month | 10 | 5 | 12 | 7 | |
| At least once per week | 19 | 9 | 15 | 8 | |
| Current or previous counseling | 174 | 84 | 151 | 85 | .75 |
| Currently taking antidepressants | 160 | 77 | 141 | 79 | .73 |
TABLE 1. Baseline characteristics of patients randomly assigned to Mindful Mood Balance plus usual depression care (MMB+UDC) or to UDC alone
During the 12-month follow-up period, patients in the MMB+UDC group had slightly higher outpatient mental health costs, mostly driven by more psychotherapy visits (cost difference of $27.05 for psychotherapy visits) (Table 2). Costs for second-generation antipsychotics were substantially higher for the MMB+UDC group, which was found to be driven by 18 more prescriptions dispensed of aripiprazole among fewer than five patients. Abilify was a high-cost medication during the study and accounted for over 90% of the costs in that category for both groups. Overall, the incremental cost, or the difference between MMB and MMB+UDC with all costs incorporated, was $431.54.
| Cost type and | UDC | MMB+UDC | Cost | ||
|---|---|---|---|---|---|
| description | N | $ | N | $ | difference ($) |
| Outpatient mental health visit | |||||
| Psychiatric | 39 | 23.32 | 40 | 27.38 | 4.06 |
| Psychotherapy | 364 | 197.22 | 432 | 224.27 | 27.05 |
| Prescription (N dispensed) | |||||
| Antidepressant | 1,132 | 1,342.80 | 933 | 1,350.97 | 8.17 |
| Second-generation antipsychotic | 26 | 74.68 | 40 | 225.05 | 150.37 |
| Anxiety and sleepa | 164 | 48.27 | 203 | 58.36 | 10.09 |
| Anticonvulsant | 92 | 160.07 | 83 | 131.18 | –28.90 |
| Stimulant (for attention disorders) | 164 | 125.67 | 93 | 138.64 | 12.97 |
| Other psychotropicb | 22 | 40.00 | 17 | 11.45 | –28.54 |
| Emergency and inpatient | |||||
| Emergency department visit | 43 | 220.44 | 39 | 363.64 | –143.20 |
| Inpatient stay | 19 | 1350.51 | 11 | 774.27 | –576.24 |
| Labor | |||||
| Recruitment | 83.48 | 83.48 | — | ||
| Coaching | — | 96.67 | 96.67 | ||
| MMB licensing | — | 180.00 | 180.00 | ||
TABLE 2. Average costs per patient during the 12-month follow-up after random assignment to Mindful Mood Balance plus usual depression care (MMB+UDC) or to UDC alone
Incremental cost per DFD gained ranged from $9.63 to $15.04 for different cost inputs (Table 3). When only MMB licensing and labor costs for recruitment and coaching were included, the incremental cost was $9.63 for each additional DFD. Negligible increases to $10.72 and $11.00 occurred when psychiatric and psychotherapy visits and antidepressants were added, which represent the highest overall service costs for this sample. A larger increase in cost per DFD ($15.04) was observed when all psychotropic costs were added, which was driven by more prescriptions dispensed of aripiprazole in the MMB+UDC group. Figure 1 shows that at $15.04 willingness to pay for an additional DFD, MMB was cost neutral.
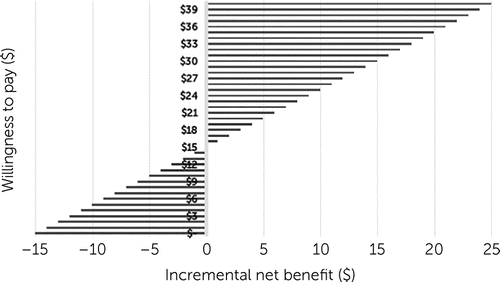
FIGURE 1. Incremental net benefit ($) at different willingness-to-pay thresholds for an additional depression-free day
| UDC cost | MMB+UDC | Incremental cost ($) | ||
|---|---|---|---|---|
| Type of costa | ($) | cost ($) | Per DFD gainedb | 95% CIc |
| Labor and MMB only | 83.48 | 360.15 | 9.63 | —d |
| Labor, MMB, and psychiatric and psychotherapy visits | 304.02 | 611.79 | 10.72 | 10.23–11.21 |
| Labor, MMB, psychiatric and psychotherapy visits, and antidepressants | 1,646.82 | 1,962.76 | 11.00 | 10.77–11.24 |
| Labor, MMB, psychiatric and psychotherapy visits, and all psychotropics | 2,095.52 | 2,527.45 | 15.04 | 13.8–16.71 |
| Labor, MMB, psychiatric and psychotherapy visits, all psychotropics, emergency department visits, and inpatient stays | 3,666.46 | 3,665.35 | –.04 | –1.32 to 1.25 |
TABLE 3. Incremental cost-effectiveness of Mindful Mood Balance (MMB), compared with usual depression care (UDC)
In the sensitivity analysis, patients who were excluded because they did not have follow-up PHQ-9 scores had negligible differences for medication and psychiatric and psychotherapy services (Table 4). Overall, this shows that those who were lost to follow-up did not seek more outpatient mental health care, but their clinical outcomes remain unknown. In the exploratory analyses, higher use of emergency and inpatient services was noted for the UDC group (17 inpatient stays for the UDC group versus 10 for the MMB+UDC group). Table 4 shows a cost savings of –$0.04 per DFD when patients lost to follow-up were eliminated from the analysis; however, the cost difference disappeared when all patients were included ($13.84 per DFD).
| Incremental cost per DFD ($)c | ||
|---|---|---|
| Type of costsb | Sensitivity | Main |
| Labor and MMB only | 9.63 | 9.63 |
| Labor, MMB, and psychiatric and psychotherapy visits | 10.37 | 10.72 |
| Labor, MMB, psychiatric and psychotherapy visits, and antidepressants | 9.09 | 11.00 |
| Labor, MMB, psychiatric and psychotherapy visits, and all psychotropics | 12.71 | 15.04 |
| Labor, MMB, psychiatric and psychotherapy visits, all psychotropics, emergency department visits, and inpatient stays | 13.84 | –.04 |
TABLE 4. Sensitivity analyses of cost-effectiveness of Mindful Mood Balance plus usual depression care (MMB+UDC) compared with UDCa
Discussion
Health insurance companies are now offering several mental health digital programs to their members as a benefit (31); however, few have undergone rigorous evaluation to understand costs (32). This cost-effectiveness study compared the MMB program to UDC by examining differences in program costs, costs for psychiatric and psychotherapy visits, and psychotropic medication costs within a large integrated health system. MMB had a cost per DFD of $9.63 for program costs only and $15.04 when all medication and services costs were included. Most of the increase over program costs only was attributed to high-cost medication choice and very little to differences in outpatient psychotherapy or psychiatric visits. Results show that MMB offers an effective and low-cost adjunctive service to traditional mental health services for treatment of residual depressive symptoms, which health systems should consider adopting.
No significant cost offsets were noted for outpatient mental health care or antidepressant medication use during the follow-up period. However, this is not surprising given the findings of prior studies of MBCT and antidepressant medications (33). Although the meditation and mindfulness skills learned in MMB may help avert use of mental health services, MMB also teaches patients to recognize warning signs to know when to engage in services, which may increase treatment seeking.
The incremental costs per patient for MMB were $431.54 for a gain of 29 DFDs. This is respectable, compared with costs of other prominent programs for depression offered through integrated health systems. For example, cost-effectiveness studies of collaborative care management for depression, which typically involves both in-person and telephone support, have indicated incremental costs of $454–$960 per patient over usual care, with 18–58 incremental DFDs gained over 1 year (32, 34–37). The incremental costs of $431.54 are comparable to those of other effective Web-based depression programs, which reported incremental costs of $52 (16), with little coaching or therapist cost but with limited engagement and costs of $703 (14) for synchronous therapist contact and high engagement. This would indicate that MMB may be equivalent to collaborative care in overall cost-effectiveness and within typical cost ranges for other Web-based depression programs with some coaching support.
The literature is sparse on robust cost estimates for in-person MBCT, compared with usual care, but two studies offered estimates of the labor cost for in-person MBCT programs. Cost per patient for an altered version called “MBCT with support to taper or discontinue antidepressant treatment” was reported to be approximately £112 ($168 in 2015 dollars) (33). An earlier study of traditional MBCT estimated that the labor costs per patient of the in-person program were the equivalent of 3 contact hours with a therapist, which would be approximately $300 based on the Medicare fee schedule (7). The equivalent to these estimates from the study reported here is coaching costs, which were $96.67 per patient over the course of MMB. Of note, in a meta-analysis, the hazard ratios (HRs) for depressive relapse were nearly the same for in-person MBCT (10) and for Web-based MMB (12) (in person MBCT, HR=0.69, 95% confidence interval [CI]=0.58–0.82; Web-based MMB, HR=0.61, 95% CI=0.39–0.95). This indicates that Web-based MMB with e-mail or phone coaching offers a substantially lower-cost alternative, compared with in-person MBCT, with nearly equivalent outcomes.
It is important to consider that patients who decide to enroll in a study for MMB may have different preferences from those choosing in-person MBCT or another in-person program, such as collaborative care for depression. Web-based MMB offers an alternative for patients with logistical barriers or social anxiety or simply for those who do not prefer in-person group-based programs (38). However, some patients may learn best in an in-person group environment, and those without stable Internet access would not be able to participate in a Web-based program. These barriers may explain why 37% of patients did not complete at least four sessions of MMB (considered an adequate dose). However, this attrition rate is lower than rates of >50% reported for prior Web-based depression programs (39). Regardless, future studies of MMB and other Web-based depression treatments should examine mediators of successful treatment response and attrition to improve delivery of these programs, especially because treatment failures and delay exacerbate the severity and chronicity of illness course (40). To address the burden of depression, it is important that multiple modalities of treatment be offered that address differences in patients’ preferences and access. This analysis indicated that MMB offers a highly feasible and scalable model for health systems looking to expand their offering of evidence-based programs for depression.
This study had several advantages and some limitations, compared with prior cost-effectiveness studies of Web-based depression programs. Many studies have not captured program costs (e.g., staff time to engage and retain patients) or have used patient self-report measures for service utilization, whereas this study captured costs associated with implementing the program and estimated actual service costs collected from EHRs and claims within a capitated health plan. One limitation is the potential for missing mental health service costs paid for privately outside the health system; however, these should be minimal because private mental health care would have higher out-of-pocket costs, compared with the covered benefits reported here. White females accounted for about three-quarters of the sample, which may affect generalizability with respect to race, ethnicity, and gender. Data for patient time costs, presenteeism, and absenteeism were not available, and thus an analysis from a societal perspective was not possible.
Another limitation was that the sample size was too small and the costs variability too high to be interpretable for emergency department and inpatient costs associated with mental health and substance abuse issues and suicide ideation and behavior. This information was included for transparency, but it is not likely accurate to say that MMB is cost-saving, considering that the inclusion of a handful of patients who were lost to follow-up changed the cost per DFD from –$0.04 to $13.84. The latter is close to the cost when only outpatient and medication costs were included ($12.71). This is also illustrated in the large cost differences associated with aripiprazole for a small additional number of administrations in the MMB group. Aripiprazole is prescribed to patients as adjunctive to antidepressants when antidepressants alone lack adequate effectiveness (41). This study was powered on the effectiveness outcomes and not on cost outcomes. Therefore, this study was not equipped to absorb these differences associated with high cost variance from emergency services, inpatient services, and high-cost medications. The total outpatient and emergency department visits, inpatient stays, and medication administrations are included in Table 2 to show proportionally that most of the utilization was associated with antidepressants and outpatient visits. When only antidepressants medications were incorporated, the incremental cost per DFD was $11.00. However, the more conservative estimate that incorporated aripiprazole is reported because it is possible that MMB caused more treatment-seeking behavior, which resulted in the additional medication administrations. This study did not report quality-adjusted life years, because this metric is rarely used by policy makers (e.g., CMS and U.S. Preventive Services Task Force) or by health system decision makers in the United States, and there is controversy about the use of this metric because of potential violation of the Americans With Disability Act (42).
Conclusions
From a health plan perspective, this study evaluated the cost-effectiveness of MMB, a Web-based program for patients with residual depressive symptoms, over a 1-year follow-up, considering all program and mental health service utilization costs. At a $0 willingness to pay, MMB would cost $15.04 per DFD. MMB is a cost-effective Web-based program for reducing residual depressive symptoms and preventing major depressive relapse, which health systems should consider adopting as adjunctive to traditional mental health care services.
1 : Depression is the leading cause of disability around the world. JAMA 2017; 317:1517Google Scholar
2 : The societal cost of depression: evidence from 10,000 Swedish patients in psychiatric care. J Affect Disord 2013; 150:790–797Crossref, Medline, Google Scholar
3 : Depression in the workplace: an economic cost analysis of depression-related productivity loss attributable to job strain and bullying. Work Stress 2013; 27:321–338Crossref, Google Scholar
4 : The costs of depression. Psychiatr Clin North Am 2012; 35:1–14Crossref, Medline, Google Scholar
5 : Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med 2010; 40:41–50Crossref, Medline, Google Scholar
6 : Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med 2011; 41:1165–1174Crossref, Medline, Google Scholar
7 : Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 2000; 68:615–623Crossref, Medline, Google Scholar
8 : Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol 2004; 72:31–40Crossref, Medline, Google Scholar
9 : The Mindful Way Through Depression: Freeing Yourself From Chronic Unhappiness. New York, Sounds True, 2007Google Scholar
10 : Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse: an individual patient data meta-analysis from randomized trials. JAMA Psychiatry 2016; 73:565–574Crossref, Medline, Google Scholar
11 : The economic burden of depression and the cost-effectiveness of treatment. Int J Methods Psychiatr Res 2003; 12:22–33Crossref, Medline, Google Scholar
12 : Outcomes of online mindfulness-based cognitive therapy for patients with residual depressive symptoms: a randomized clinical trial. JAMA Psychiatry 2020; 77:563–573Crossref, Medline, Google Scholar
13 : Cost-effectiveness of computerised cognitive-behavioural therapy for anxiety and depression in primary care: randomised controlled trial. Br J Psychiatry 2004; 185:55–62Crossref, Medline, Google Scholar
14 : Cost-effectiveness of therapist-delivered online cognitive-behavioural therapy for depression: randomised controlled trial. Br J Psychiatry 2010; 197:297–304Crossref, Medline, Google Scholar
15 : Preventing depression in adults with subthreshold depression: health-economic evaluation alongside a pragmatic randomized controlled trial of a Web-based intervention. J Med Internet Res 2017; 19:e5Crossref, Medline, Google Scholar
16 : Clinical and cost-effectiveness of therapist-guided internet-delivered cognitive behavior therapy for older adults with symptoms of depression: a randomized controlled trial. Behav Ther 2015; 46:193–205Crossref, Medline, Google Scholar
17 : Cognitive behavior therapy via the Internet: a systematic review of applications, clinical efficacy and cost-effectiveness. Expert Rev Pharmacoecon Outcomes Res 2012; 12:745–764Crossref, Medline, Google Scholar
18 Integrating Behavioral Health Into Accountable Care Organizations: Challenges, Successes, and Failures at the Federal and State Levels. Alexandria, VA, National Association of State Mental Health Directors, 2016. https://www.nasmhpd.org/sites/default/files/Assessment%207_Integrating%20Behavioral%20Health%20 into%20ACOs.pdfGoogle Scholar
19 : The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613Crossref, Medline, Google Scholar
20 : Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ 2012; 184:E191–E196Crossref, Medline, Google Scholar
21 : Advantages of using estimated depression-free days for evaluating treatment efficacy. Psychiatr Serv 2010; 61:160–163Link, Google Scholar
22 : Costing behavioral interventions: a practical guide to enhance translation. Ann Behav Med 2009; 37:218–227Crossref, Medline, Google Scholar
23 : Economic analyses of the Be Fit Be Well program: a weight loss program for community health centers. J Gen Intern Med 2013; 28:1581–1588Crossref, Medline, Google Scholar
24 Occupational Employment and Wages, May 2020: 21-1091: Health Education Specialists. Washington, DC, US Department of Labor, Bureau of Labor Statistics, 2021. https://www.bls.gov/oes/current/oes211091.htm. Accessed Oct 20, 2019Google Scholar
25 : A standardized relative resource cost model for medical care: application to cancer control programs. J Natl Cancer Inst Monogr 2013; 2013:106–116Crossref, Medline, Google Scholar
26 : Medical care costs for recurrent versus de novo stage IV cancer by age at diagnosis. Health Serv Res 2018; 53:5106–5128Crossref, Medline, Google Scholar
27 Measuring Price Change in the CPI: Medical Care. Washington, DC, US Department of Labor, Bureau of Labor Statistics, 2021. https://www.bls.gov/cpi/factsheets/medical-care.htmGoogle Scholar
28 : Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res 2018; 53:175–196Crossref, Medline, Google Scholar
29 : Uncertainty of incremental cost-effectiveness ratios. a comparison of Fieller and bootstrap confidence intervals. Int J Technol Assess Health Care 1999; 15:608–614Crossref, Medline, Google Scholar
30 : Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002; 23:377–401Crossref, Medline, Google Scholar
31 : Digital tools are revolutionizing mental health care in the US. Harvard Bus Rev, Dec 3, 2020. https://hbr.org/2020/12/digital-tools-are-revolutionizing-mental-health-care-in-the-u-sGoogle Scholar
32 : Mental health apps: what to tell patients. Curr Psychiatr 2018; 17:21–25Google Scholar
33 : Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet 2015; 386:63–73Crossref, Medline, Google Scholar
34 : Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry 2001; 158:1638–1644Link, Google Scholar
35 : Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry 2005; 62:1313–1320Crossref, Medline, Google Scholar
36 : Cost-effectiveness of practice-initiated quality improvement for depression: results of a randomized controlled trial. JAMA 2001; 286:1325–1330Crossref, Medline, Google Scholar
37 : Cost-effectiveness of treatments for major depression in primary care practice. Arch Gen Psychiatry 1998; 55:645–651Crossref, Medline, Google Scholar
38 : Web-based intervention in mindfulness meditation for reducing residual depressive symptoms and relapse prophylaxis: a qualitative study. J Med Internet Res 2014; 16:e87Crossref, Medline, Google Scholar
39 : Dropout rates in clinical trials of smartphone apps for depressive symptoms: a systematic review and meta-analysis. J Affect Disord 2020; 263:413–419Crossref, Medline, Google Scholar
40 : Functional recovery in major depressive disorder: focus on early optimized treatment. Prim Care Companion CNS Disord 2016; 18(5). doi: 10.4088/PCC.15r01926Medline, Google Scholar
41 : A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom 2012; 81:87–97Crossref, Medline, Google Scholar
42 Quality-Adjusted Life Years and the Devaluation of Life With Disability: Part of the Bioethics and Disability Series. Washington, DC, National Council on Disability, Nov 6, 2019. https://ncd.gov/sites/default/files/NCD_Quality_Adjusted_Life_Report_508.pdfGoogle Scholar



