Implementation Science in Thailand: Design and Methods of a Geriatric Mental Health Cluster-Randomized Trial
Abstract
Background:
Thailand has a rapidly aging population yet lacks evidence for effective and scalable evidence-based psychosocial interventions to support persons living with dementia and their family caregivers. In this study of a culturally adapted and evidence-based clinical program (Reducing Disabilities in Alzheimer’s Disease [RDAD]), designed to reduce behavioral and psychological symptoms of dementia in older adults, the authors test the hypothesis that an implementation support strategy, Getting To Outcomes (GTO), would produce better implementation and clinical outcomes compared with usual implementation of RDAD in Thailand.
Methods:
The study uses a hybrid type III cluster-randomized design to compare eight geographical districts that receive training on both implementing the RDAD clinical intervention and on GTO implementation support strategies (intervention arm) with eight other districts that receive the same RDAD training but without training in GTO implementation support strategies (control arm). GTO is an evidence-based intervention designed to support implementers to better plan, implement, and evaluate innovative intervention programs in a novel setting. Primary outcomes, including implementation and clinical outcomes, will be assessed at baseline, month 3 (posttreatment), and month 6 (3-month follow-up).
Results:
The research team anticipates that there will be significantly more improvements in the delivery of the RDAD intervention program in the experimental group than in the control group.
Next steps:
If clinical trial findings are positive, the authors plan to replicate and scale up the proposed implementation science approach across Thailand to enhance and expand mental health services for older adults with dementia.
Like many other low- and middle-income countries (LMICs) in Asia, Thailand is facing a dramatic increase in the number of older persons with dementia in the coming decades (1). However, very little evidence exists to guide policy makers in developing programs and supports for older adults living with dementia and their family caregivers (2). This project represents Thailand’s first effort to develop a scale-up implementation research study to close the mental health treatment gap for older adults with any mental health condition. This project primarily focuses on the reduction of behavioral and psychological symptoms of dementia (BPSD), which are present in 50% to 100% of persons with dementia and are associated with many adverse outcomes, including increased disability and reduced quality of life for the individual, as well as more stress, burden, and reduced quality of life for family caregivers (3).
Based on the observation that many prior government-promoted initiatives have failed to be fully accepted, implemented, and sustained by local practitioners, this study focuses on testing whether an evidence-based implementation support strategy, Getting To Outcomes (GTO) (4), leads to better implementation and clinical outcomes for the Reducing Disabilities in Alzheimer’s Disease (RDAD) intervention (see Hypotheses below). GTO, an intervention protocol designed to provide implementation support for administrations to better plan, implement, and evaluate evidence-based intervention programs, has been found to increase the capacity of organizations to implement a variety of evidence-based programs, particularly in improving fidelity and performance of implementation (4, 5). Given that RDAD has been found to reduce BPSD in both experimental settings (6) and community-based residential settings (7), we will rely on a type III hybrid study design, which primarily tests implementation support strategies, with a secondary aim of evaluating clinical effectiveness (8, 9).
Given the shortages of mental health specialists, insufficient financing for mental health services, and accumulating evidence for using trained nonspecialists to deliver mental health care (i.e., the task-sharing approach) (10), we plan to implement RDAD, a culturally adapted version of an evidence-based intervention model (11), in both study arms. RDAD combines physical exercise and behavioral management for older adults with dementia and BPSD, relying on community-based health workers with additional training in elder care—i.e., care managers (CMs) and community caregivers (CCGs)—for program delivery in Thailand.
Methods
Study Design
The study will be implemented in 16 geographical districts (i.e., clusters), and all districts will be randomly assigned to either the intervention arm or the control arm. Both arms will receive the same RDAD clinical intervention protocol. The intervention arm will receive the RDAD intervention plus the GTO implementation support protocol, while the control arm will receive RDAD only.
The intervention study period will last for 6 months, providing adequate time to observe effects. Patient and caregiver data will be collected at baseline, month 3 (posttreatment), and month 6 (3-month follow-up) (Figure 1).
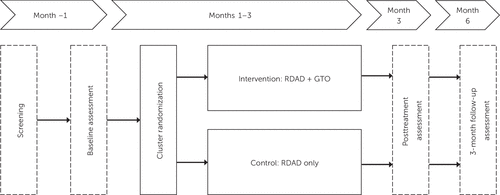
FIGURE 1. Basic design of the randomized controlled trial
Study Sites
This study will be conducted in Thailand’s Khon Kaen province, which has 26 districts; each district comprises from three to 18 subdistricts. Each subdistrict has one long-term care (LTC) program led by a CM (typically a trained nurse) along with several CCGs (typically community health workers with additional training in elder care) who provide health and social care to community-residing elders. Ten of the 26 districts were excluded because they were already participating in another dementia-related project (N=2), their health care systems were too new to be fully functioning (N=4), and there were too few active LTC programs to support feasibility of the intervention within the LTC system (N=4). Of the remaining 16 districts (147 subdistricts, with over 267,000 older adults), eight were randomly selected for the experimental arm and the other eight for the control arm.
Inclusion and Exclusion Criteria
Patients will be included in the study if they are age 60 or above, have “probable dementia,” have one or more behavioral or psychological symptoms, are ambulatory, and have an adult (age ≥18) family caregiver who lives with or spends at least 4 hours every day with them. Measures used to determine eligibility include the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), a locally validated short questionnaire completed by a family relative or friend familiar with the person to assess cognitive impairment in older adults (score ≥3.47 indicates probable dementia) (13); the Neuropsychiatric Inventory (NPI-Q), a well-validated instrument translated into Thai that relies on caregiver self-report to assess the presence and severity of BPSD (14); a validated ambulatory test that assesses a person’s ability to walk independently or with an assistive device (e.g., canes or walkers) but without physical assistance for support from another person (5); and a medical history constructed from family caregivers’ responses about the presence of chronic diseases, with any positive response further evaluated by the study physician to identify persons with medical contradictions to physical exercise who should be excluded from study participation. Patients will be excluded from the study if they or their family caregivers do not assent to study participation or if their CM and/or primary care providers recommend that the patient not participate in the intervention because of concerns about a medical condition (e.g., severe or unstable cardiovascular disease) and/or frailty based on medical record.
Recruitment Procedures
Patients will be primarily recruited from referrals from Thailand’s LTC system, where participating CMs identify candidate study participants based on their Mini-Mental State Examination (MMSE) scores, which are routinely collected and included in patients’ LTC plans. LTC patients who have been identified as having cognitive impairment on the basis of the MMSE will be screened with the IQCODE to identify persons at high risk for dementia, referred to hereafter as “probable dementia.” Participants with an IQCODE mean score above the 3.47 threshold for probable dementia and who demonstrate at least one significant BPSD symptom as measured by the NPI-Q will be eligible for participation in the trial. On the basis of the high sensitivity (90%) and specificity (95%) of the IQCODE (15), we anticipate that the positive predictive value of the IQCODE will be >90%, thus ensuring the likelihood that we will successfully recruit the required sample in the proposed study period.
Case Confirmation
Because many persons with dementia in Thailand will not have received a formal diagnosis and because project resources do not allow us to obtain a clinical diagnosis, we will rely on an adjudication process to identify persons with probable dementia. After the initial screening based on IQCODE is completed, a team consisting of the research assistant, research director, CM, and study physician will review the results of the IQCODE, along with other medical conditions (e.g., cardiac disease, hypertension), to determine whether a person meets clinical criteria for dementia according to DSM-5 and to identify any medical contraindications to the exercise intervention.
Power Analysis
Achieving 80% power for a medium effect (0.4) at an alpha level of 0.05 among 16 districts/clusters will require 288 dyads (each comprising a person with dementia and a family caregiver) for the whole study. We plan to recruit 340 (170 per group) dyads, allowing for 15% attrition.
Consent Procedures
Two separate consents will be obtained: one for participation in screening and one for participation in the intervention study. Elderly individuals who screen positive on the assessments (i.e., IQCODE and NPI-Q) will be eligible for participation in the randomized controlled trail (RCT). Per Thai institutional review board (IRB) laws, both the family caregiver and the person with dementia are required to consent to the participation of the person with dementia in the trial. The family caregiver, or someone who has the right to act as the legally authorized representative of the person with dementia (e.g., legal guardian or family member), will provide a signature to indicate permission for his or her relative’s study participation. If the family caregiver is not able to read and write, a thumbprint from the family caregiver is required to indicate permission for the person with dementia to participate.
The person with dementia will be read a consent form that uses simple language to explain the study. We will follow recommendations of Black and colleagues (16) for assisting persons whose capacity for consent might be diminished. Thus, study staff are trained to observe verbal willingness to participate (e.g., saying yes or being willing to do what the family caregiver consents to), behavioral indications (e.g., acting agreeable, cooperating), and emotional indications (e.g., having a positive facial expression) (16). They will also observe indications of dissent to participate, as expressed verbally (e.g., saying no), behaviorally (e.g., not cooperating, being agitated), or emotionally (e.g., showing distress or unhappiness) (16).
Persons with dementia who do not provide verbal, behavioral, or emotional dissent will provide consent through either a signature or thumbprint to confirm their agreement for study participation. Persons with dementia have fluctuating behavioral and psychological problems (e.g., agitation, apathy, paranoia) that might temporarily interfere with their ability to engage in the consent process. Thus, if they are not able to provide consent by either signature or thumbprint, they will be approached up to two additional times. If they are not able to consent after three attempts, they will not be eligible for study participation. Although the Thai procedure differs from U.S. IRB study procedures, which rely on an “assent process” facilitated by an expert assessing the capacity of the person with dementia for assent, the proposed procedure for those with diminished capacity to consent is compliant with Thai IRB laws as well as the applicant institution’s IRB.
In addition, consent will be obtained from family caregivers for their own participation in the RCT intervention study. Although caregivers are not required to participate in the intervention’s physical exercise activities, they will be actively engaged in helping ensure the safety of the person with dementia throughout the physical activity intervention. The family caregiver will also be engaged in the behavioral aspects of the intervention study, learning how to better manage BPSD. Similar to prior interventions showing reductions in stress and burden and increased quality of life for involved caregivers, this study will document and analyze changes in family caregiver outcomes.
Randomization Procedure
Cluster randomization will occur at the district level. To maximize the even distribution of factors that may affect outcomes (17), we employed three steps to implement the cluster randomization procedure. First, we conducted a formative evaluation of geographical variation among districts in Khon Kaen province and identified two covariates that might affect implementation outcomes: number of subdistricts (range 4 to 18) and the ratio of active LTC systems to number of LTC systems, as discussed above. Second, we stratified all participating sites/districts by these two covariates for a 2×2 distribution of districts, resulting in an even distribution across the four conditions. We next conducted stratified randomization, using a computer-generated randomization program to randomly assign the sites in sequence to ensure equal distribution between two study arms.
Intervention Protocols
The intervention and control arms will receive the same culturally adapted and evidence-based RDAD clinical intervention, in accordance with its standard implementation guidelines, as well as the “usual practices” of program implementation in Thailand. Usual implementation practice consists of a top-down centralized program announcement, with a printed description of the program and its administrative requirements (e.g., schedule for submission of “care plans” and other performance indicators) and basic consultation support from district LTC management offices. The intervention arm will receive the additional GTO intervention to support implementation of the RDAD protocol (Table 1).
| RDAD intervention activities | GTO intervention activities | |
|---|---|---|
| Week | (experimental and control arms) | (experimental arm only) |
| –1 | No activity | Session 1: TA skills (group) training to TA specialists; overview of GTO steps 1–10 (3-day workshop) |
| 0 | Session 0: CM in control arm (group) training on RDAD (2-day workshop) | Session 2: CM (group) training on RDAD and GTO steps 1–6; TAs help sites complete tools for step 1–6 (3-day workshop) |
| 1 | Session 1: home visit; session 2: home visit | Session 3: onsite TA supporting CM on quality implementation |
| 2 | Session 3: home visit; session 4: home visit | Session 4: onsite TA supporting CM on quality implementation |
| 3 | Session 5: home visit; session 6: home visit | Session 5: onsite TA supporting CM on quality implementation |
| 4 | Session 7: home visit; session 8: home visit | Session 6: onsite TA supporting CM on quality implementation |
| 5 | Session 9: home visit | Session 7: TA skills (group) training to TA specialists; intensive training of GTO steps 7–9 and lessons learned in 5 weeks of TA (2-day workshop) |
| 6 | Session 10: home visit | Session 8: CM (group) training on GTO steps 7–9; TAs help sites complete tools for step 7–9 (2-day workshop) |
| 7 | Session 11: home visit | Session 9: onsite TA supporting CM on quality implementation |
| 8 | Session 12: home visit | Session 9: onsite TA supporting CM on quality implementation |
| 9 | No activity | No activity |
| 10 | No activity | No activity |
| 11 | No activity | Session 10: TA skills (group) training to TA specialists; intensive training of GTO steps 10 and lessons learned in 11 weeks of TA (2-day workshop) |
| 12 | Session 13: home visit | Session 11: CM (group) training on GTO steps 10; TAs help sites complete tools for step 10 (2-day workshop) |
TABLE 1. Intervention activities in the Reducing Disabilities in Alzheimer’s Disease intervention studya
RDAD intervention.
The RDAD protocol was part of the Seattle Protocols originated by Teri and colleagues (6, 7, 18) in a series of clinical trials. The RDAD protocol includes two main components. The behavioral management component involves teaching family caregivers how to identify and modify behavioral problems of the patient that impair day-to-day function and adversely affect patient-caregiver interactions; providing information about dementia, nutrition support, support with home environment adaptation, and support for enhancing family caregiver coping skills; and identifying pleasant activities in which family caregivers can engage (6, 7). The physical exercise component includes aerobic and endurance activities and strength, balance, and flexibility training, with the goal of engaging patients in physical exercise for a minimum of 30 minutes a day (19).
GTO intervention.
GTO is an evidence-based intervention to support the systematic planning, implementation, and evaluation of innovative interventions (defined as new practices, policies, or procedures), such as RDAD, in novel settings (20). The two core components of GTO include training GTO technical assistance specialists to provide implementation support to CMs and training CMs to follow the GTO protocol for high-quality implementation. Through a 10-step process (i.e., assessing needs, developing goals and desired outcomes, selecting a best practice, ensuring program fit, ensuring sufficient capacity, planning, process evaluation, outcome evaluation, continuous quality improvement, and sustainability), GTO provides a detailed roadmap for those seeking to implement evidence-based interventions customized to their communities.
We hypothesize that the implementation support provided through GTO will increase capacity for all key programming activities, including goal setting, strategic planning, monitoring, continuous quality improvement, and sustainability. As a result, better performance of programming activities will improve program delivery, which in turn will lead to better individual outcomes (20). The delivery of GTO training and GTO-based implementation support will be performed throughout the 3-month intervention period (Table 1) to support implementation of the RDAD clinical intervention.
Feasibility and Cultural Adaptation
To assess the feasibility of conducting the proposed intervention study in Thailand, we conducted five formal meetings with over 30 key stakeholders (including health policy makers, different types of health service providers, researchers, and care receivers) and in-depth qualitative interviews with eight health professionals. We made site visits to community-based primary care settings and residential homes (Chuengsatiansup et al., 2020, unpublished manuscript). The focus of these meetings was to determine the most appropriate service delivery system in which to implement the study.
Themes and key messages that emerged from these meetings were examined, and follow-up conversations were held to clarify issues that were raised. The research team gradually achieved consensus regarding three items. First, there is increased recognition of burden of care for older adults with BPSD. Second, there are system-wide shortages of mental health specialists in the LTC system and limited availability of trained community-based health professionals in the LTC system to deliver in-home support services to persons with dementia (Box 1). Third, intervention programs should be delivered by community-based health professionals in the existing service platform of the Thai LTC system.
BOX 1. Key challenges, advantages, and design solutions in the Reducing Disabilities in Alzheimer&s Disease (RDAD) intervention study
Key Challenges
Shortages of mental health specialists, in general, and geriatric mental health specialists, in particular Insufficient financing for mental health services Lack of infrastructure to support scale-up of successful or promising programs
Key Advantages
Increased recognition of population aging and its associated burden of care for older adults with behavioral and psychological symptoms of dementia (BPSD)
Availability of newly organized long-term-care (LTC) system, with trained care managers (CMs) and community caregivers (CCGs) providing in-home support for persons with dementia Availability of evidence-based clinical intervention programs and implementation programs
Design Solutions
Develop an intervention program to be delivered by community-based care providers (i.e., CMs and CCGs in the existing service platform of the Thai LTC system) who are familiar with the target population and ready to implement the program
Develop an implementation support mechanism by training implementation technical assistance specialists in each participating district to support care providers in implementing RDAD
Use a stage III hybrid design to primarily test the GTO implementation support strategy, with a secondary aim to evaluate clinical outcomes of the RDAD intervention for persons with BPSD and their caregivers
Through the formative research, the original RDAD protocol was modified for local cultural, clinical, and service systems in a two-step process. First, possible modifications were explored in collaboration with RDAD’s developer during her consultation with the study team in Thailand. The research team then held a series of workshops with CMs and CCGs to obtain their feedback about the acceptability of the recommended cultural adaptations, the feasibility of implementing them for Thai culture, and any additional changes that they recommended to ensure cultural and clinically appropriate communication of key concepts. The following adaptations to the original protocol were agreed upon: using local devices (e.g., drums) and activities (e.g., walking to a Buddhist temple, local folk dance) to illustrate acceptable physical activities, using storytelling to elicit CMs’ and CCGs’ clinical experiences to increase their appreciation of the relevance of the intervention programs to their clinical practice, and shortening intervals between follow-up sessions (the original 5-month RDAD intervention was changed to 12 weeks, which matches the reporting and monitoring cycle of the local LTC service system). All of these adaptations were approved by the RDAD developer, who confirmed that all critical elements of the intervention were preserved.
The proposed protocol will be implemented over a 12-week period, for a total of 13 sessions. Each dyad will be seen in the patient’s home for all 13 sessions, with each session lasting from approximately 30 minutes to 1 hour. Besides in-person discussion at each session, a brochure showing physical activity procedures and a set of forms (e.g., appointment schedule, RDAD procedures checklist) will also be given to the family caregivers. The schedule of home visits is as follows: eight sessions (2 sessions per week) for the first 4 weeks (weeks 1–4), followed by four sessions (1 session per week) for 4 weeks (weeks 5–8), and one last session on week 12. After the first nine home visit sessions, which are primarily for teaching, coaching, and reviewing, four additional follow-up home visit sessions will occur to review the status of behavioral management practice and physical exercise, answer questions, consolidate treatment gains, and encourage long-term retention in the program.
Training for Intervention Delivery
We conducted workshops with CMs and CCGs to ensure understanding of key concepts of the GTO and RDAD intervention protocols and to ensure that they fit the clinical workflow in the Thai context. Workshops were followed by qualitative interviews with trainees. We also conducted consensus meetings in which the research team, including clinicians, medical anthropologists, and public health officials, worked closely with participating CMs and CCGs to determine the fit of the interventions within the existing clinical flow of the Thai LTC system.
Hypotheses
Given that GTO has been shown to increase implementers’ overall capacity to implement evidence-based intervention programs, our primary hypothesis is that the intervention arm, which employs the GTO implementation support intervention, will result in significantly better implementation outcomes. Based on the prior literature (see below), we selected the following five implementation outcome indicators: higher fidelity in implementing the RDAD clinical intervention, higher dosage of RDAD received by the participants, better reach of participants in the community, better acceptability as perceived by the RDAD program implementers, and higher satisfaction of the participants (i.e., family caregivers and persons with BPSD) with the RDAD clinical intervention, as compared with the control arm.
Assuming that improved implementation of the RDAD clinical intervention will lead to better intervention outcomes, our secondary hypothesis is that the intervention arm will lead to better clinical outcomes for persons with BPSD, including greater reduction in BPSD, better general medical health, and improved overall quality of life, compared with persons with BPSD in the control arm. In addition, because the family caregiver is also a key receiver of the RDAD intervention while assisting persons with BPSD, our tertiary hypothesis is that the intervention arm will lead to better family caregiver outcomes, including reduced burden, lower psychological distress, and improved quality of life, compared with caregivers in the control arm.
Outcome Measures
Three sets of measures will be used for outcome evaluation (Table 2). Implementation outcomes (primary objective) include fidelity (the extent to which the content of the intervention is delivered as intended), assessed by percentage of scheduled sessions being completed, based on a study log documented by the family caregiver after each session and confirmed by CCGs weekly (10); dosage (the actual amount of intervention received by the participants), assessed by CCGs and documented in a study log (7); reach assessment, based on records from interventionists (i.e., CM and CCG) and administrators and documented in a study log (21); acceptability, assessed by CCG and CM responses to a brief questionnaire at months 3 and 6 (22); satisfaction, assessed by the family caregiver and person with dementia (if possible) in response to questionnaire administered at months 3 and 6 (23); and readiness for change, assessed by the Activity-Based Readiness Tool, a self-report questionnaire to be completed by CCGs and CMs at baseline, month 3, and month 6 (24).
| Instrument | Source (reference no.) | Respondent | Testing occasion |
|---|---|---|---|
| Implementation outcomes | |||
| Fidelity | Teri et al., 2003 (6) | CCG | Intervention |
| Dosage | Teri et al., 2003 (6) | CCG or CM | Intervention |
| Reach | Proctor et al., 2011 (21) | CCG and CM | T2, T3 |
| Acceptability | Weiner et al., 2017 (22) | CCG or CM | T2, T3 |
| Satisfaction | Meyers et al., 2012 (23) | CCG | T2, T3 |
| Activity-Based Readiness Tool | Wandersman and Scaccia, 2018 (24) | CCG or CM | T1, T2, T3 |
| PwD clinical outcomes | |||
| IQCODE | Senanarong et al., 2001 (13) | Caregiver | Screening |
| NPI-Q, symptom score | Cummings et al., 1994 (14) | Caregiver | Screening, T1, T2, T3 |
| Ambulatory Walk Test | McCurry et al., 2018 (11) | PwD | Screening |
| NPI-Q, all scores | Cummings et al., 1994 (14) | Caregiver | T1, T2, T3 |
| Geriatric Depression Scale | Yesavage et al., 1982 (25) | Caregiver | T1, T2, T3 |
| SF-36 (2 domains: physical functioning and physical role functioning) | Ware and Sherbourne, 1992 (27) | Caregiver | T1, T2, T3 |
| Sickness Impact Profile, mobility subscale | Bergner et al., 1981 (28) | Caregiver | T1, T2, T3 |
| Barthel ADL Index | Collin et al., 1988 (29) | PwD | T1, T2, T3 |
| QOL-AD (PwD) | Logsdon et al., 1999 (30) | PwD | T1, T2, T3 |
| TMSE | Train the Brain Forum Committee, 1993 (26) | PwD | T1 |
| Caregiver clinical outcomes | |||
| Zarit Burden Interview | Bédard et al., 2001 (31) | Caregiver | T1, T2, T3 |
| PHQ-8 | Kroenke et al., 2001 (32) | Caregiver | T1, T2, T3 |
| NPI-Q, caregiver distress score | Cummings et al., 1994 (14) | Caregiver | T1, T2, T3 |
| QOL-AD (caregiver) | Logsdon et al., 1999 (30) | Caregiver | T1, T2, T3 |
TABLE 2. Summary of key measures in the Reducing Disabilities in Alzheimer’s Disease intervention studya
Patient clinical outcomes (secondary objective), collected at baseline, 3 months (posttreatment), and 6-month follow-up, include behavioral and psychological symptoms, assessed by the NPI-Q (14); depressive symptoms, assessed by the Geriatric Depression Scale (25); cognitive impairment, assessed by the Thai Mental State Examination (TMSE) (26); physical functioning, assessed by the Medical Outcomes Study Short Form Health Survey–36 (27) and the Sickness Impact Profile (mobility subscale) (28); activities of daily living, assessed by the Barthel Index for Activities of Daily Living (29); and quality of life, assessed by Quality of Life in Alzheimer Disease (QoL-AD) (30). All of these scales, except for the TMSE, are based on caregiver report.
Caregiver clinical outcomes (tertiary objective), collected at baseline, month 3, and month 6, include caregiver burden, assessed by the Zarit Burden Interview (31); depressive symptoms, assessed by the Patient Health Questionnaire–8 (PHQ-8) (32) (i.e., PHQ-9 without the suicidality item); caregiver distress, assessed by symptom-related distress on the NPI-Q (14); and quality of life, assessed by QoL-AD for caregivers (30).
Data Analysis
In addition to descriptive analyses and univariate comparisons of the experimental and control intervention group conditions at baseline, we will use repeated-measures mixed models to analyze each of the implementation and clinical outcome measures. We will also use an intraclass coefficient to reflect the expected positive within-group correlation. For the analysis, the outcomes will be calculated for each individual participant, and individual outcomes data will be nested within each district. Using an intention-to-treat approach, we will conduct analysis of covariance with multivariate linear regression modeling to test effects of the intervention at month 3 and month 6. Intercepts and treatment effect will be modeled as random effects at the participant level and as fixed effects at the district level, whereas patient characteristics (e.g., levels of dementia severity and mobility) as well as length of caregiver involvement (i.e., average hours spent per day) will be control variables. Results of statistical procedures will be presented by using adjusted means (least-squares means) with 95% confidence intervals for intervention and control groups, respectively.
Anticipated Results
We anticipate that over the 3- to 6-month intervention period, there will be significantly more improvements in the delivery of the RDAD intervention program, as indexed by implementation outcome measures (i.e., fidelity, dosage, acceptability, satisfaction), in the experimental group than in the control group. Furthermore, we anticipate that patients in the experimental arm will demonstrate significantly more improvement in their mental and general medical health and that caregivers will report more reduction in both caregiver burden and stress than those in the control arm. These findings, together with other published studies that have used a similar approach, will form a convincing empirical foundation for policy makers and clinical leaders in Thailand to consider not only utilizing an evidence-based intervention to improve care for older adults with BPSD but also utilizing the GTO implementation support strategy to ensure success of intervention program delivery.
This clinical trial is the first large-scale implementation intervention study in Thailand with a primary focus on evaluating the benefit of utilizing implementation science methodology for successful implementation of clinical programs. In LMICs such as Thailand, where mental health professional resources are limited, it is crucial to identify evidence-based intervention programs that can be culturally adapted and implemented using the available workforce in the existing service system and that can be scaled up with the support necessary for expanding service capacity. Our approach, delivering an intervention to address a mental health challenge by relying primarily on existing community-based health care resources (e.g., CMs, CCGs, and family caregivers) with minimal support of participating medical professionals (e.g., physicians and psychiatrists), provides a promising message about how to deliver a feasible mental health treatment solution in an LMIC. The implementation support strategies, through GTO protocol-based interactive training and use of trained technical assistance specialists for support, may inform the current practice and add new knowledge to the research on implementation science.
Next Steps
If this study is successful, its findings can be used to further the agenda of expanding mental health care for older adults in Thailand. We will first provide the eight control districts with the combined RDAD and GTO programs, a requirement of our Thai collaborators, to ensure that control district participants feel they are being treated equally. Second, we will introduce the GTO-based implementation support model to other regional or national policy makers, employing the technical assistance specialists trained for this research project as peer trainers to build similar implementation support capacity in other regions to enhance further scaling up of the RDAD program. Third, we will work with interested investigators and policy makers to assist them in building capacity to implement other types of evidence-based mental health clinical intervention programs with GTO implementation support.
Highlights
This study is the first large-scale research in Thailand with a primary focus on examining the benefit of utilizing implementation science methodology for successful implementation of evidence-based clinical programs.
The clinical trial evaluates both implementation and clinical outcomes of implementation strategies to support the delivery of a clinical program designed to reduce behavioral and psychological symptoms of dementia in older adults.
Findings from this study can be used to further the agenda of expanding mental health care for older adults in Thailand.
1 : World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London, Alzheimer’s Disease International, 2015Google Scholar
2 : Interventions to support family caregivers of people living with dementia in high, middle and low-income countries in Asia: a scoping review. BMJ Glob Health 2019; 4:e001830Crossref, Medline, Google Scholar
3 : Prevalence of neuropsychiatric symptoms in Alzheimer’s disease: a cross-sectional descriptive study in Thailand. J Med Assoc Thai 2014; 97:560–565Medline, Google Scholar
4 : Intervening with practitioners to improve the quality of prevention: one-year findings from a randomized trial of assets-getting to outcomes. J Prim Prev 2013; 34:173–191Crossref, Medline, Google Scholar
5 : An intervention to improve program implementation: findings from a two-year cluster randomized trial of Assets Getting To Outcomes. Implement Sci 2013; 8:87Crossref, Medline, Google Scholar
6 : Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA 2003; 290:2015–2022Crossref, Medline, Google Scholar
7 : Translating an evidence-based multicomponent intervention for older adults with dementia and caregivers. Gerontologist 2020; 60:548–557Crossref, Medline, Google Scholar
8 : Effectiveness -implementation hybrid designs: implications for quality improvement science. Implement Sci 2013; 8:S2Crossref, Medline, Google Scholar
9 : Reenvisioning clinical science: unifying the discipline to improve the public health. Clin Psychol Sci 2014; 2:22–34Crossref, Medline, Google Scholar
10 : The acceptability and feasibility of task-sharing for mental healthcare in low and middle income countries: a systematic review. Soc Sci Med 2013; 97:82–86Crossref, Medline, Google Scholar
11 : Training area agencies on aging case managers to improve physical function, mood, and behavior in persons with dementia and caregivers: examples from the RDAD-Northwest Study. J Gerontol Soc Work 2018; 61:45–60Crossref, Medline, Google Scholar
13 : The IQCODE: an alternative screening test for dementia for low educated Thai elderly. J Med Assoc Thai 2001; 84:648–655Medline, Google Scholar
14 : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
15 : Modified Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia for Thai elderly. Southeast Asian J Trop Med Public Health 2006; 37:587–594Medline, Google Scholar
16 : Seeking assent and respecting dissent in dementia research. Am J Geriatr Psychiatry 2010; 18:77–85Crossref, Medline, Google Scholar
17 : The method of randomization for cluster-randomized trials: challenges of including patients with multiple chronic conditions. Int J Stat Med Res 2016; 5:2–7Crossref, Medline, Google Scholar
18 : Exercise interventions for dementia and cognitive impairment: the Seattle Protocols. J Nutr Health Aging 2008; 12:391–394Crossref, Medline, Google Scholar
19 : A randomized controlled clinical trial of the Seattle Protocol for Activity in older adults. J Am Geriatr Soc 2011; 59:1188–1196Crossref, Medline, Google Scholar
20 : Early stages of development of a peer specialist fidelity measure. Psychiatr Rehabil J 2016; 39:256–265Crossref, Medline, Google Scholar
21 : Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011; 38:65–76Crossref, Medline, Google Scholar
22 : Psychometric assessment of three newly developed implementation outcome measures. Implement Sci 2017; 12:108Crossref, Medline, Google Scholar
23 : The quality implementation framework: a synthesis of critical steps in the implementation process. Am J Community Psychol 2012; 50:462–480Crossref, Medline, Google Scholar
24 : Readiness Briefing Paper: FY 2017–2018. Columbia, SC, Wandersman Center, 2018Google Scholar
25 : Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17:37–49Crossref, Medline, Google Scholar
26
27 : The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30:473–483Crossref, Medline, Google Scholar
28 : The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981; 19:787–805Crossref, Medline, Google Scholar
29 : The Barthel ADL Index: a reliability study. Int Disabil Stud 1988; 10:61–63Crossref, Medline, Google Scholar
30 : Assessment of agitation in Alzheimer’s disease: the agitated behavior in dementia scale. Alzheimer’s Disease Cooperative Study. J Am Geriatr Soc 1999; 47:1354–1358Crossref, Medline, Google Scholar
31 : The Zarit Burden Interview: a new short version and screening version. Gerontologist 2001; 41:652–657Crossref, Medline, Google Scholar
32 : The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613Crossref, Medline, Google Scholar



