Cost-Effectiveness of Esketamine Nasal Spray for Patients With Treatment-Resistant Depression in the United States
Abstract
Objective:
This study aimed to estimate the cost-effectiveness of esketamine, a novel intranasally dosed antidepressant, for patients in the United States with treatment-resistant depression.
Methods:
A decision-analytic model parameterized with efficacy data from phase 3 randomized trials of esketamine was used to simulate the effects of treatment with esketamine versus oral antidepressants over a 5-year horizon, from both societal and health care sector perspectives. Outcomes included remission and response of depression, quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs) for esketamine. Value-based prices were calculated, defined as the per-dose price at which esketamine would become cost-effective given cost-effectiveness thresholds of $50,000/QALY, $100,000/QALY, and $150,000/QALY. Uncertainty in these outcomes was assessed with probabilistic sensitivity analyses. Key model parameters included the efficacy of esketamine versus oral antidepressants (relative risk of 1.39 for remission; 1.32 for response) and the monthly cost of esketamine ($5,572 for month 1; $1,699–$2,244 thereafter).
Results:
Over 5 years, esketamine was projected to increase time in remission from 25.3% to 31.1% of life-years, resulting in a gain of 0.07 QALYs. Esketamine increased societal costs by $16,617 and health care sector costs by $16,995. Base case ICERs were $237,111/QALY (societal) and $242,496/QALY (health care sector). Probabilistic sensitivity analysis showed a greater than 95% likelihood that esketamine’s ICER would be above $150,000/QALY. At a cost-effectiveness threshold of $150,000/QALY, esketamine’s value-based price was approximately $140/dose (versus a current price of $240/dose).
Conclusions:
Esketamine is unlikely to be cost-effective for management of treatment-resistant depression in the United States unless its price falls by more than 40%.
HIGHLIGHTS
On the basis of several phase 3 randomized, controlled trials demonstrating its efficacy, esketamine nasal spray was recently approved by the United States Food and Drug Administration for management of treatment-resistant depression.
Using data from these trials and a decision-analytic model, the authors estimated a greater than 95% likelihood that intranasal esketamine would not be cost-effective in the United States, according to commonly applied standards.
For esketamine to become cost-effective, its price would need to fall to $140 or less per dose, a greater than 40% decline from its current price of approximately $240 per dose.
Esketamine is an intranasally dosed antidepressant that was approved by the United States Food and Drug Administration (FDA) in March 2019 for use in treatment-resistant major depressive disorder (1). Its approval has sparked intense interest for several reasons.
First, esketamine has a novel mechanism of action (NMDA receptor antagonism) compared with prior antidepressants, which act primarily via monoamine neurotransmitter modulation (2). Second, esketamine’s rapid onset of effect has raised the potential for novel applications, such as acute reduction of suicidality in emergency settings (3). Third, and the reason for its FDA approval, esketamine has demonstrated efficacy in patients with treatment-resistant depression (4), that is, patients for whom two or more prior treatments have failed (5). Patients with treatment-resistant depression experience markedly reduced quality of life and increased health care costs (6) and have remission rates with further antidepressants as low as 10% to 15% (7). As a novel treatment option with demonstrated efficacy in this subpopulation, esketamine could thus have immense individual and societal benefits.
However, esketamine has drawbacks that may limit its use. Most notably, the FDA requires that patients be observed in a health care setting for 2 hours after each dose to monitor for dissociation and hypertension (1). Even without considering the costs of this monitoring, esketamine is substantially more expensive than other antidepressants. The first 2 months of esketamine treatment have been estimated to cost $7,000 to $11,000 (8); in contrast, a recent assessment of national price databases revealed that commonly used oral antidepressants cost less than $100 per year (9, 10). Amid ongoing efforts to control the growth of health care spending in the United States, it is critical to ensure that such an expensive treatment provides a good health-economic value (11, 12).
To address this issue, we used a previously developed decision-analytic model (13) integrating data from four recently published phase 3 clinical trials (4, 14) in order to assess the value of esketamine. Our analysis had two main objectives: first, to quantify the cost-effectiveness of esketamine nasal spray compared with usual care for patients with treatment-resistant depression in the United States; and second, to determine drug price thresholds at which esketamine would be considered cost-effective according to commonly applied criteria in the United States.
Methods
Overview
We used a decision-analytic model parameterized with data from phase 3 clinical trials of esketamine to simulate the clinical and economic consequences of the following two treatment strategies for adults with major depressive disorder for whom two prior antidepressants have failed: esketamine (initial treatment with esketamine nasal spray plus oral antidepressants, followed by additional antidepressants and/or psychotherapy if depressive symptoms do not respond) and usual care (initial treatment with oral antidepressants, followed by additional antidepressants and/or psychotherapy if depressive symptoms do not respond).
For each strategy, we projected symptomatic outcomes, quality-adjusted life-years (QALYs), and costs, in 2015 U.S. dollars, over a 5-year time horizon. We chose this time horizon to ensure adequate time for longer-term costs and benefits to accrue without requiring excess extrapolation beyond available outcomes data (4, 15, 16); we varied the time horizon between 2 and 10 years in sensitivity analyses. Future costs and QALYs were discounted at an annual rate of 3% (17). We evaluated costs from both the health care sector perspective (incorporating those costs accrued within the medical system) and the societal perspective (including additional costs such as productivity losses and patient time) (17). (An impact inventory describing these perspectives is available in an online supplement to this article.)
Using these outcomes, we calculated the incremental cost-effectiveness ratio (ICER) of esketamine as the ratio of its incremental cost to its incremental QALYs relative to usual care; a lower ICER indicates a better health-economic value. In the United States, ICER thresholds between $50,000/QALY and $150,000/QALY have been advocated for designating health interventions as “cost-effective” (18, 19). To reflect this range of recommendations, we report results for distinct cost-effectiveness thresholds of $50,000/QALY, $100,000/QALY, and $150,000/QALY. For each threshold, we calculated the value-based price of esketamine, defined as the per-dose price at which its ICER is equal to the threshold (20); this value represents the highest price at which esketamine would be deemed cost-effective for a given cost-effectiveness threshold.
In reporting our methods and findings, we adhered to the 2013 Consolidated Health Economic Evaluation Reporting Standards guidelines (21). Because this study used published literature without identifiable data, it was exempt from institutional review board regulation. The study was conducted between March 2019 and December 2019.
Model Description
To simulate the effects of esketamine, we used a previously developed state-transition model of depression treatment (10, 13). The model uses a 1-month cycle length. The model is implemented in Microsoft Excel; statistical analyses were performed with OpenMetaAnalyst (22).
Patients enter the model upon initiation of third-line antidepressant treatment and can progress through up to nine total treatment lines (see figure in online supplement). With the exception of esketamine, these treatment lines do not simulate specific medications, psychotherapy, or other treatments; they are instead intended to capture the aggregate costs and effectiveness of the range of therapies provided to patients with treatment-resistant depression (7, 9).
Each treatment line is modeled using five health states: initiation, the first month of receiving a new treatment; remission, near-complete resolution of depressive symptoms, as determined by a validated symptom rating scale, for example, a score of ≤5 on the Quick Inventory of Depressive Symptomatology (7); response, partial resolution of depressive symptoms, defined as a reduction of 50% or more in the score on a validated symptom rating scale (7, 14); nonresponse, initial failure to achieve response or remission; and relapse, the recurrence of depressive symptoms after initial response or remission.
Patients start each new treatment in the initiation state and then transition to remission, response, or nonresponse in the next cycle on the basis of efficacy estimates for that treatment. Patients in remission and response are then subject to a monthly probability of relapse. Patients in relapse or nonresponse will transition to initiation of the following treatment line during the next cycle. Patients who never respond to a given treatment will receive it for 8 weeks (4 weeks in initiation, 4 weeks in nonresponse), consistent with recommendations for the duration of an adequate antidepressant trial (23, 24).
Finally, patients in all states are subject to a monthly all-cause mortality probability; for simplicity, this probability is not shown in the model diagram (see the online supplement).
Model Input Data
Base case model input data, uncertainty analysis ranges, and sources are shown in Table 1. Where possible, input data reflect U.S. adults with treatment-resistant, nonpsychotic major depressive disorder.
| Parameter | Base case value | Uncertainty analysis range | Distribution | Source |
|---|---|---|---|---|
| General and demographic | ||||
| Annual discount rate (%) | 3 | Sanders et al. (17) | ||
| Time horizon (years) | 5 | 2–10a | ||
| Annual mortality probability (%) | .40 | .38–.43 | Normal | Amos et al. (25), Arias et al. (26), Cuijpers et al. (27) |
| Esketamine efficacy relative to usual care | Janssen (4) | |||
| Response (relative risk) | 1.32 | 1.10–1.58 | Log-normal | |
| Remission (relative risk) | 1.39 | 1.09–1.79 | Log-normal | |
| Relapse (hazard ratio) | 1 | |||
| Usual care efficacy by treatment line | Rush et al. (7) | |||
| Remission probability (%) | ||||
| Line 3 | 30.6 | 28.2–33.0 | Beta | |
| Line 4 | 13.7 | 10.4–17.2 | Beta | |
| Lines 5+ | 13.0 | 7.7–19.5 | Beta | |
| Response probability (%) | ||||
| Line 3 | 28.5 | 26.2–30.9 | Beta | |
| Line 4 | 16.8 | 13.4–20.8 | Beta | |
| Lines 5+ | 16.3 | 10.3–23.2 | Beta | |
| Monthly relapse probability (%) | ||||
| In remission, line 3 | 7.8 | 6.6–9.1 | Beta | |
| In remission, line 4 | 7.7 | 4.1–12.6 | Beta | |
| In remission, lines 5+ | 8.6 | 3.7–16.4 | Beta | |
| In response, line 3 | 13.6 | 11.4–16.1 | Beta | |
| In response, line 4 | 17.8 | 12.9–24.2 | Beta | |
| In response, lines 5+ | 17.3 | 11.0–27.4 | Beta | |
| Utility by health state | Sapin et al. (30) | |||
| Nonresponse, relapse, initiation | .58 | .50–.66 | Normal | |
| Response | .72 | .65–.79 | Normal | |
| Remission | .85 | .83–.87 | Normal | |
| Costs (2015 $) | ||||
| Annual direct health care cost by treatment line | Amos et al. (25) | |||
| Line 3 | 12,047 | 9,666–14,428 | Normal | |
| Line 4 | 14,699 | 11,441–17,957 | Normal | |
| Line 5 | 15,073 | 13,053–17,093 | Normal | |
| Line 6 | 16,699 | 13,939–19,459 | Normal | |
| Lines 7+ | 18,667 | 17,089–20,245 | Normal | |
| Annual lost productivity cost by health state | Stewart et al. (38) and Bureau of Labor Statistics (39) | |||
| Nonresponse, relapse, initiation | 11,573 | 8,063–15,083 | Normal | |
| Response | 5,757 | 3,445–8,070 | Normal | |
| Remission | 2,067 | 1,338–2,796 | Normal | |
| Monthly costs of esketamine treatment | ||||
| Medication costs | Janssen (4) and U.S. Department of Veterans Affairs (34) | |||
| Month 1 | 5,572 | |||
| Months 2+, response | 2,244 | |||
| Months 2+, remission | 1,699 | |||
| Months 2+, nonresponse, relapse | 1,966 | |||
| Physician and medical assistant services | Janssen (4), Centers for Medicare and Medicaid Services (36), and Bureau of Labor Statistics (37) | |||
| Month 1 | 685 | |||
| Months 2+, response | 275 | |||
| Months 2+, remission | 218 | |||
| Months 2+, nonresponse, relapse | 246 | |||
| Patient time | Janssen (4) and Bureau of Labor Statistics (39) | |||
| Month 1 | 689 | |||
| Months 2+, response | 276 | |||
| Months 2+, remission | 219 | |||
| Months 2+, nonresponse, relapse | 248 |
TABLE 1. Input data in a decision-analytic model simulating effects of treatment with esketamine versus usual care among patients with treatment-resistant depression
Demographics and mortality.
On the basis of demographic data from an insurance claims analysis of patients with treatment-resistant depression, we simulated a population with mean±SD age of 40.5±13.2 years in which 64.2% were female (25). We applied age- and gender-specific mortality rates from the 2013 U.S. life tables (26), along with a mortality hazard ratio of 1.58 (95% confidence interval [CI]=1.47–1.70) for people with depression versus the general population (27); this yielded an overall annual mortality probability of 0.0040.
Esketamine efficacy.
We based our estimates of esketamine’s efficacy on the results of four phase 3 clinical trials that are presented in the FDA Advisory Committee briefing document for esketamine (4). In three short-term trials (TRANSFORM-1, TRANSFORM-2, and TRANSFORM-3), patients with major depressive disorder for whom two or more prior treatment lines had failed were randomly assigned to receive esketamine nasal spray versus placebo; in addition, all patients received a new oral antidepressant. TRANSFORM-1 and TRANSFORM-2 included patients age 18 to 64 years; TRANSFORM-3 included patients age 65 years or older. We performed random effects, restricted maximum likelihood meta-analyses on 4-week intention-to-treat remission and response rates from these trials (see figures in online supplement). Of 421 total patients randomly assigned to receive esketamine, 142 (34%) achieved remission, and 199 (47%) achieved response; of 289 patients randomly assigned to receive placebo, 68 (24%) achieved remission, and 102 (35%) achieved response. Estimated relative risks for esketamine versus placebo were 1.39 (95% CI=1.09–1.79) for remission and 1.32 (95% CI=1.10–1.58) for response.
In the long-term SUSTAIN-1 trial, patients who achieved initial remission or response when receiving esketamine were randomly assigned to continuation of esketamine versus replacement with placebo (4). During follow-up extending up to 89 weeks, relapse hazard ratios for esketamine versus placebo were 0.49 (95% CI=0.29–0.84) for patients in remission at random assignment and 0.30 (95% CI=0.16–0.55) for patients in response. These results indicate that continuation of esketamine reduced relapse risk compared with withdrawal of esketamine among patients who initially responded to it; they do not indicate that patients who responded to esketamine had a lower relapse risk than patients who responded to oral antidepressants. Consequently, in the base case of our analysis we assumed equal relapse rates for esketamine and oral antidepressants; we varied this assumption in sensitivity analysis.
Usual care efficacy.
Estimated rates of remission (30.6% to 13.0%) and response (28.5% to 16.3%) across multiple treatment lines under usual care were drawn from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial (7). STAR*D was a pragmatic randomized trial in which outpatients with major depressive disorder were followed across four treatment “steps.” Specific treatments included citalopram, bupropion, cognitive therapy, lithium augmentation, and others. Notably, remission and response rates in the first step of STAR*D (36.8% and 48.6%, respectively) were lower than observed in multiple meta-analyses of antidepressant efficacy (28, 29), possibly due to prior treatment experience among STAR*D participants; for this reason, we derived our estimates of third-line treatment efficacy from step 2 of STAR*D.
Monthly relapse probabilities (5.1% to 17.8%) were derived from the long-term follow-up phase of STAR*D (7). Observed relapse rates were higher for patients in response than in remission and for patients who had received more prior treatments.
Utility.
Utility values for our model’s depression health states were derived from a French study that used the Euro-Qol-5D questionnaire to assess quality of life among outpatients treated for major depressive disorder; utility estimates for remission, response, and nonresponse were 0.85, 0.72, and 0.58 (30).
Costs.
Cost estimates are expressed in 2015 U.S. dollars. We used the Medical Care Expenditure index from the Bureau of Labor Services to inflate costs from earlier years to 2015 (the most recent year for which this index was available) (31, 32). We deflated later costs to 2015 by using the Personal Consumption Expenditure index from the Federal Reserve (33).
We derived estimates of the aggregate cost of depression treatment (including medications and outpatient, inpatient, and emergency department services) from a claims-based analysis of privately insured patients with major depressive disorder in the United States. (25). Annual costs ranged from $12,047 to $18,667 for patients with two prior treatments to patients with six or more prior treatments.
Our estimate of the total cost of esketamine provision included costs of the medication itself, physician visits, and medical assistant observation during the 2 hours after each dose. We used a cost per 28 mg of esketamine of $240, drawn from the Veterans Affairs Federal Supply Schedule (34); of note, this database tends to provide a lower-bound estimate for a medication’s cost (35). Per manufacturer recommendations, esketamine is given twice weekly for the first 4 weeks and weekly or every other week thereafter; the initial dose is 56 mg, with subsequent doses either 56 mg or 84 mg (4). We used observed dose and dose-frequency data from the TRANSFORM-2 and SUSTAIN-1 trials (4) to estimate the number of doses patients would receive in the first month and subsequent months, depending on depression health state (see table in online supplement). Multiplying by the above unit cost yielded monthly medication costs ranging from $5,572 for month 1 to $1,699 for patients in remission after month 1.
We assumed that patients would see a physician at each presentation for esketamine dosing; we used a cost of $71 per visit, based on reimbursement from the 2019 Centers for Medicare and Medicaid Services Physician Fee Schedule for a 15-minute office visit (CPT code 99213) (36). Finally, we assumed a medical assistant would supervise four patients per 2-hour dosing and monitoring period, resulting in a per-patient cost of $8 for 30 minutes of medical assistant time (37).
When performing analyses from the societal perspective, we included additional nonmedical costs. We used severity-dependent productivity losses of 8.4 hours/week, 4.2 hours/week, and 1.5 hours/week for patients in the nonresponse, response, and remission states, derived from a national survey-based study of American workers with depression (38). We applied a cost of $26/hour to these productivity losses on the basis of average hourly earnings in the United States (39). Finally, we assumed each esketamine dose would require 3 hours of patient time (including dosing, monitoring, and travel) with a cost of $26/hour (39).
Uncertainty and Sensitivity Analyses
We performed several types of sensitivity analysis to quantify the impact of modeling assumptions and uncertainty in model input parameters on our results. In one-way sensitivity analyses, we varied the values of individual model parameters (or groups of parameters) and assessed their effect on our results. All parameters in Table 1 were included in one-way sensitivity analyses except for discount rate, relapse rate with esketamine, and esketamine costs, which were assessed in separate sensitivity analyses.
In probabilistic sensitivity analyses, the model was run using parameter values drawn at random from distributions reflecting their uncertainty (Table 1). Model results were compiled across 10,000 repetitions of this process in order to quantify the aggregate uncertainty in results attributable to joint uncertainty in the model’s input parameters.
Finally, in scenario sensitivity analyses, we assessed the effects of specific alternative modeling assumptions on our results. We included the following scenarios: excluding TRANSFORM-3 from our meta-analysis of esketamine efficacy due to the older patient population in this trial (see figures in online supplement) (4); incorporating improved relapse rates with esketamine versus usual care, based on SUSTAIN-1, which reported relapse hazard ratios of 0.49 (remitters) and 0.30 (responders) (4); providing esketamine as a fourth-line or fifth-line treatment, rather than third-line; providing electroconvulsive therapy after failure of third-line treatment (electroconvulsive therapy efficacy parameters were 50.9% remission, 66.6% response, and 3.0% relapse per month; monthly costs were $5,077 for the first month and $846 thereafter) (13); eliminating costs of physician visits, medical assistant time, and patient time from the total cost of esketamine to assess the effect of relaxing monitoring requirements.
Results
Model Validation
To assess the external validity of our model, we compared model output to independent estimates from the literature. We identified two relevant validation targets for clinical outcomes. In a cohort with treatment-resistant depression in the United Kingdom followed prospectively for 8 to 84 months (mean=39 months), patients spent an average of 39.9% of the time with uncontrolled symptoms (that is, not in remission or response) (16). In contrast, in a cohort with treatment-resistant depression in the United States followed for 24 months, patients spent 87.8% of the time with uncontrolled symptoms (15). Over 32 months of usual care, our model projected that patients spend 61.7% of the time with uncontrolled symptoms.
Regarding economic outcomes, a 2018 systematic review identified seven U.S.-based studies on the costs of treatment-resistant depression; annual health care costs ranged from $12,000 to $19,000 (6). For comparison, over 24 months of usual care (the most common follow-up duration in the included studies), our model projected an annual health care cost of $15,949.
Five-Year Health and Economic Outcomes
Base case results are shown in Table 2. Over 5 years, esketamine was projected to increase the fraction of time patients are in remission from 25.3% to 31.1%; this translated to a gain of 0.07 QALYs. Total costs were projected to increase by $16,617 from the societal perspective and $16,995 from the health care sector perspective. This cost increase was driven largely by the cost of esketamine itself ($16,352), with smaller contributions from physician and medical assistant service costs ($2,062) and patient time costs ($2,074). Other health care costs and lost productivity costs both declined with esketamine. Esketamine was not cost-effective under either perspective, with projected ICERs of $237,111/QALY (societal) and $242,496/QALY (health care sector).
| Parameter | Usual care | Esketamine | Difference |
|---|---|---|---|
| Time spent in health state (%) | |||
| Initiation, nonresponse, relapse | 72.3 | 66.9 | –5.4 |
| Response | 2.4 | 2.0 | −.4 |
| Remission | 25.3 | 31.1 | 5.7 |
| QALYsa | 3.00 | 3.07 | .07 |
| Total costs (2015 $) | |||
| Societal perspective | 121,073 | 137,690 | 16,617 |
| Health care sector perspective | 79,786 | 96,781 | 16,995 |
| Cost components (2015 $) | |||
| Esketamine treatment | |||
| Medication | 16,352 | 16,352 | |
| Physician and medical assistant services | 2,062 | 2,062 | |
| Patient time | 2,074 | 2,074 | |
| Other health care costs | 79,786 | 78,367 | –1,419 |
| Lost productivity | 41,287 | 38,836 | –2,451 |
| Incremental cost-effectiveness ratios ($/QALY) | |||
| Societal perspective | 237,111 | ||
| Health care sector perspective | 242,496 |
TABLE 2. Base case results of a decision-analytic model simulating effects of treatment with esketamine versus usual care among patients with treatment-resistant depression
Uncertainty and Sensitivity Analyses
In one-way sensitivity analyses, esketamine’s ICER did not fall below $150,000/QALY with variation in any individual parameter (see figure in online supplement). The lowest ICER attained with any parameter variation was $182,770/QALY (societal perspective), when applying the 95% CI upper limit estimates of relative risk of remission and response with esketamine versus usual care.
In probabilistic sensitivity analyses, the probability that esketamine is cost-effective was less than 0.01 at cost-effectiveness thresholds of $50,000/QALY and $100,000/QALY, regardless of perspective. At a threshold of $150,000/QALY, the probability that esketamine is cost-effective was 0.04 under a societal perspective and 0.02 under a health care sector perspective.
Value-based price estimates, along with confidence intervals based on probabilistic sensitivity analyses, are shown in Figure 1. To be cost-effective at a threshold of $150,000/QALY from the societal perspective, the per-dose price of esketamine would need to fall from its current value of $240 to a price of $144 or below (95% CI=$24–$253). From the health care sector perspective, the per-dose price of esketamine would need to fall to $141 or below (95% CI=$42–$237).
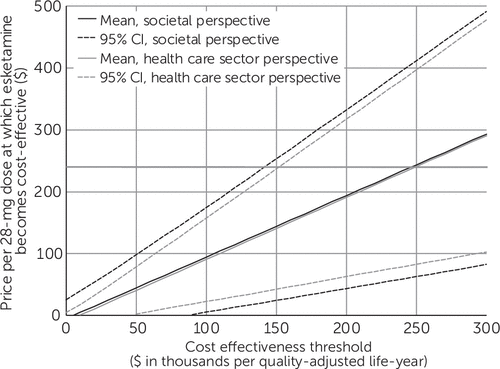
FIGURE 1. Value-based price estimates for esketaminea
aCI, confidence interval. The horizontal axis indicates the cost-effectiveness threshold used to classify an intervention as cost-effective or not. Plotted curves show the price per 28-mg dose at which esketamine would achieve an incremental cost-effectiveness ratio equal to the threshold (that is, the value-based price) along with confidence intervals estimated via probabilistic sensitivity analysis. The horizontal line indicates the current price per 28-mg dose of esketamine ($240).
In scenario sensitivity analyses (Figure 2), esketamine required price reductions to become cost-effective under all scenarios except one. At a cost-effectiveness threshold of $150,000/QALY from the societal perspective, esketamine’s value-based price was $244 when we simulated 50% to 70% reductions in relapse rate with esketamine versus usual care.
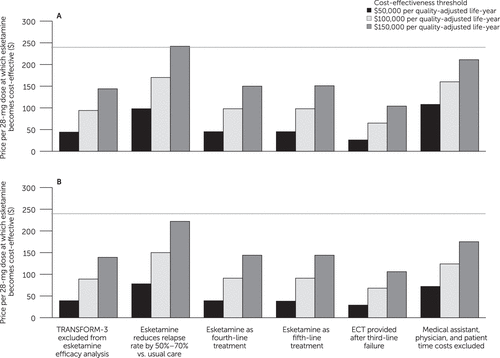
FIGURE 2. Scenario sensitivity analyses for the value-based price of esketamine, from (A) the societal perspective and (B) the health care sector perspectivea
aECT, electroconvulsive therapy. The vertical axis indicates the value-based price of esketamine, that is, the price per 28-mg dose below which esketamine would be deemed cost-effective at a given cost-effectiveness threshold. Bars indicate value-based prices at three alternative cost-effectiveness thresholds. For comparison, the horizontal line indicates the current price per 28-mg dose of esketamine ($240). The horizontal axis shows six scenarios simulated in sensitivity analysis, consisting of alternative modeling and input parameter assumptions related to treatment efficacy, treatment costs, and alternative treatments.
Discussion
In this decision-analytic modeling analysis incorporating recently released phase 3 clinical trial data (4), we found that esketamine nasal spray is unlikely to be a cost-effective treatment for patients with treatment-resistant depression in the United States at a cost of approximately $240 per dose. Our model projected base case ICERs of $237,111/QALY (societal perspective) and $242,496/QALY (health care sector perspective) for esketamine, compared with usual care. In uncertainty analysis, we found a greater than 95% likelihood that esketamine’s ICER would fall above $150,000/QALY, a commonly applied upper bound for defining cost-effective medical interventions in the United States (18). We estimated that esketamine would require a price reduction of more than 40% from its current price of approximately $240 per 28-mg dose (to approximately $140 or less per dose) to become cost-effective (34).
Our results were consistent across a wide range of deterministic and probabilistic sensitivity analyses. Of note, we did identify a single scenario under which esketamine’s ICER was below $150,000/QALY: when we assumed 50% to 70% reductions in depression relapse rate with esketamine versus usual care. However, as described in the Methods section, this assumption represents an inappropriate application of findings from the SUSTAIN-1 trial (4) and was included only as an exploratory analysis. Under all other sensitivity analyses, including optimistic assumptions about esketamine’s cost and efficacy, we found that esketamine cannot be considered cost-effective.
Two prior studies have evaluated the cost-effectiveness of esketamine. An evidence report released by the Institute for Clinical and Economic Review estimated esketamine’s ICER to be $198,000/QALY (40), consistent with our finding that esketamine is unlikely to be cost-effective. In contrast, an industry-supported conference abstract reported an ICER below $100,000/QALY; a more precise value was not provided in the abstract (41).
From a health policy perspective, esketamine’s current status—as a clinically effective medication that is priced well above its value-based price—is far from unique (11). For example, in a recent assessment of the 50 drugs accounting for the greatest New York State Medicaid spending, five had value-based price estimates; of these, two were priced at or below their value-based price, and the remaining three required discounts of up to 54% to reach their value-based price (12). Still, targeted efforts by payers and policy makers can be effective in reducing prices, even for drugs with market exclusivity. In the recent case of evolocumab and alirocumab, two novel lipid-lowering agents approved by the FDA in 2015 and initially priced at approximately $14,000/year, insurers’ efforts at limiting access via restrictive prior authorizations and copayment garnered 60% reductions in the medications’ prices by 2018 (42). Subsequent analyses showed that the medications now meet cost-effectiveness criteria (42, 43). However, these price reductions came at the expense of curtailing use of these medications. Prior to the price reductions, less than 1% of eligible patients received prescriptions for these medications (44), and a third of patients abandoned their prescriptions when confronted with the copay amount (45). In the case of esketamine, we are hopeful that policy makers and insurers can use positive incentives and proactive negotiation to establish fair prices without making the drug inaccessible to patients with treatment-resistant depression who could benefit from it (11, 12, 46, 47).
For individual patients and providers, the cost of esketamine (in terms of out-of-pocket expenses, patient time, or costs to the health care system as a whole) is likely to be an important component of shared decision-making (48). Fortunately, there are multiple alternative options for treatment-resistant depression that have been shown to be cost-effective. Until esketamine’s price falls, we would recommend considering electroconvulsive therapy (13), antipsychotic augmentation (49), or cognitive-behavioral therapy (50, 51).
Our results should be interpreted in the context of several limitations. It is important to recognize that the data used to simulate effects of esketamine reflect samples of a few hundred clinical trial participants (4); longer-term observational data or additional randomized trials could refine our cost-effectiveness estimates. In light of these limitations in input data, our model employs a 5-year time horizon, which may fail to capture relevant longer-term health-economic consequences. Reassuringly, however, cost-effectiveness outcomes were robust to variation in time horizon. Next, there were several limitations to the data drawn from other sources. In instances where inadequate data were available from patients with treatment-resistant depression, we have derived model inputs from the overall population with major depressive disorder, which may result in underestimating the degree of occupational impairment and mortality in treatment-resistant individuals (52, 53). Our utility estimates for remission, response, and nonresponse were drawn from a French study and thus may be less applicable to the United States (30). Reassuringly, however, these utility estimates are consistent with those obtained from an international meta-analysis (54). Our estimates of depression severity-dependent productivity losses are not disaggregated by gender, which may lead to some inaccuracy given the overrepresentation of women among people with major depressive disorder (25, 38). Finally, in the absence of comparative outcomes data, we were unable to directly assess esketamine’s cost-effectiveness versus alternatives such as electroconvulsive therapy or transcranial magnetic stimulation; however, indirect comparison in scenario sensitivity analysis revealed that our findings were unchanged when electroconvulsive therapy was included as a comparator.
Conclusions
In this decision-analytic modeling analysis, we found that esketamine is unlikely to be cost-effective for management of treatment-resistant depression at its current price of $240 per dose. Esketamine could become cost-effective by U.S. standards if its price were to fall to $140 or less per dose. Achieving these price reductions while ensuring continued access for the patients who stand to benefit from esketamine will require careful and concerted efforts from payers and policy makers.
1 : Esketamine for treatment-resistant depression—first FDA-approved antidepressant in a new class. N Engl J Med 2019; 381:1–4Crossref, Medline, Google Scholar
2 : What is new about new antidepressants? Psychother Psychosom 2018; 87:129–139Crossref, Medline, Google Scholar
3 : Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 2018; 175:620–630Link, Google Scholar
4 Advisory Committee Briefing Document: Esketamine Nasal Spray for Patients With Treatment-Resistant Depression. Raritan, NJ, Janssen Research & Development, 2019. https://www.fda.gov/media/121377/download. Accessed Sept 6, 2019Google Scholar
5 : Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry 2017; 74:9–10Crossref, Medline, Google Scholar
6 : The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord 2019; 242:195–210Crossref, Medline, Google Scholar
7 : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Link, Google Scholar
8 : Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA (Epub ahead of print, Aug 2, 2019)Google Scholar
9 : Antidepressant medication prescribing practices for treatment of major depressive disorder. Psychiatr Serv 2017; 68:199–202Link, Google Scholar
10 : The cost-effectiveness of cognitive behavioral therapy versus second-generation antidepressants for initial treatment of major depressive disorder in the United States: a decision analytic model. Ann Intern Med 2019; 171:785–795Crossref, Medline, Google Scholar
11 : The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA 2016; 316:858–871Crossref, Medline, Google Scholar
12 : Value-based pricing and state reform of prescription drug costs. JAMA 2017; 318:609–610Crossref, Medline, Google Scholar
13 : Cost-effectiveness of electroconvulsive therapy vs pharmacotherapy/psychotherapy for treatment-resistant depression in the United States. JAMA Psychiatry 2018; 75:713–722Crossref, Medline, Google Scholar
14 : Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry 2019; 176:428–438Link, Google Scholar
15 : Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry 2006; 67:688–695Crossref, Medline, Google Scholar
16 : Prediction of longer-term outcome of treatment-resistant depression in tertiary care. Br J Psychiatry 2012; 201:369–375Crossref, Medline, Google Scholar
17 : Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016; 316:1093–1103Crossref, Medline, Google Scholar
18 : ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2304–2322Crossref, Medline, Google Scholar
19 : Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014; 371:796–797Crossref, Medline, Google Scholar
20 : Value-based pricing for drugs: theme and variations. JAMA 2018; 319:2165–2166Crossref, Medline, Google Scholar
21 : Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluations Publication Good Reporting Practices Task Force. Value Health 2013; 16:231–250Crossref, Medline, Google Scholar
22 : Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 2012; 49:1–15Crossref, Google Scholar
23 : Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. Can J Psychiatry 2016; 61:540–560Crossref, Medline, Google Scholar
24 : Florida best practice psychotherapeutic medication guidelines for adults with major depressive disorder. J Clin Psychiatry 2017; 78:703–713Crossref, Medline, Google Scholar
25 : Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a US commercial claims database. J Clin Psychiatry 2018; 79:1–9Crossref, Google Scholar
26 : United States life tables, 2013. Natl Vital Stat Rep 2017; 66:1–64Google Scholar
27 : Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry 2014; 171:453–462Link, Google Scholar
28 : Re-evaluation of the efficacy and tolerability of venlafaxine vs SSRI: meta-analysis. Psychopharmacology (Berl) 2008; 196:511–520, discussion 521–522Crossref, Medline, Google Scholar
29 : A meta-analysis of clinical trials comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. J Psychopharmacol 2008; 22:843–848Crossref, Medline, Google Scholar
30 : Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health Qual Life Outcomes 2004; 2:20Crossref, Medline, Google Scholar
31 : Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res 2018; 53:175–196Crossref, Medline, Google Scholar
32 Medical Care Expenditure Indices From MEPS. Suitland, MD, Bureau of Economic Analysis, 2019. https://www.bea.gov/national/health_care_satellite_account.htm. Accessed Sept 5, 2019Google Scholar
33 Personal Consumption Expenditures. St. Louis, Federal Reserve Bank of St. Louis, 2019. https://fred.stlouisfed.org/series/DPCERG3A086NBEA. Accessed Aug 19, 2019Google Scholar
34 Pharmaceutical Prices. Washington, DC, US Department of Veterans Affairs Office of Procurement, Acquisition and Logistics, 2019. https://www.va.gov/opal/nac/fss/pharmPrices.asp. Accessed Sept 6, 2019Google Scholar
35 : A transparent and consistent approach to assess US outpatient drug costs for use in cost-effectiveness analyses. Value Health 2018; 21:677–684Crossref, Medline, Google Scholar
36 Physician Fee Schedule. Baltimore, Centers for Medicare and Medicaid Services, 2019. https://www.cms.gov/apps/physician-fee-schedule/ Accessed Sept 5, 2019Google Scholar
37 Occupational Outlook Handbook: Medical Assistants. Washington, DC, Bureau of Labor Statistics, 2019. https://www.bls.gov/ooh/healthcare/medical-assistants.htm. Accessed Sept 6, 2019Google Scholar
38 : Cost of lost productive work time among US workers with depression. JAMA 2003; 289:3135–3144Crossref, Medline, Google Scholar
39 Current and Real (Constant 1982–1984 Dollars) Earnings for All Employees on Private Nonfarm Payrolls, Seasonally Adjusted. Washington, DC, Bureau of Labor Statistics, 2019. https://www.bls.gov/news.release/realer.t01.htm. Accessed Sept 6, 2019Google Scholar
40 Atlas S, Agboola F, Fazioli K, et al: Esketamine for the Treatment of Treatment-Resistant Depression: Effectiveness and Value. Boston, Institute for Clinical and Economic Review, 2019. https://icer-review.org/material/trd-final-evidence-report-and-meeting-summary/. Accessed Sept 9, 2019Google Scholar
41 Hernandez LG, Li S, Toro-Diaz H, et al: Cost-effectiveness analysis of esketamine in treatment-resistant depression in the United States. Value Health 2019; 22:S228–S229Google Scholar
42 : Increasing the adoption and diffusion of a novel pharmacological therapy that is both mortality reducing and cost-effective. J Am Heart Assoc 2019; 8:
43 : Updated cost-effectiveness analysis of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease. JAMA Cardiol 2019; 4:691–695Crossref, Medline, Google Scholar
44 : Prescribing patterns of proprotein convertase subtilisin-kexin type 9 inhibitors in eligible patients with clinical atherosclerotic cardiovascular disease or heterozygous familial hypercholesterolemia. Am J Cardiol 2018; 121:1155–1161Crossref, Medline, Google Scholar
45 : Association of prior authorization and out-of-pocket costs with patient access to PCSK9 inhibitor therapy. JAMA Cardiol 2017; 2:1217–1225Crossref, Medline, Google Scholar
46 : Changing Medicare’s protected classes: history, mistrust, and budgets in the prescription drug market. Psychiatr Serv 2019; 70:858–859Link, Google Scholar
47 : Payer and policy maker steps to support value-based pricing for drugs. JAMA 2015; 314:2503–2504Crossref, Medline, Google Scholar
48 : Discussing out-of-pocket expenses during clinical appointments: an observational study of patient-psychiatrist interactions. Psychiatr Serv 2017; 68:610–617Link, Google Scholar
49 : Comparing cost-effectiveness of aripiprazole augmentation with other “next-step” depression treatment strategies: a randomized clinical trial. J Clin Psychiatry 2018; 80:1–8Crossref, Google Scholar
50 : Long-term effectiveness and cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: follow-up of the CoBalT randomised controlled trial. Lancet Psychiatry 2016; 3:137–144Crossref, Medline, Google Scholar
51 : Cost-utility analyses of cognitive-behavioural therapy of depression: a systematic review. Psychother Psychosom 2015; 84:6–21Crossref, Medline, Google Scholar
52 : All-cause mortality in patients with treatment-resistant depression: a cohort study in the US population. Ann Gen Psychiatry 2019; 18:23. https://doi.org/10.1186/s12991-019-0248-0Google Scholar
53 : Treatment-resistant depression in primary care across Canada. Can J Psychiatry 2014; 59:349–357Crossref, Medline, Google Scholar
54 : A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr Serv 2014; 65:977–987Link, Google Scholar



