Randomized Trial of a Mobile Personal Health Record for Behavioral Health Homes
Abstract
Objective:
Behavioral health homes, which provide onsite primary medical care in mental health clinics, face challenges in integrating information across multiple health records. This study tested whether a mobile personal health record application improved quality of medical care for individuals treated in these settings.
Methods:
This randomized study enrolled 311 participants with a serious mental illness and one or more cardiometabolic risk factors across two behavioral health homes to receive a mobile personal health record application (N=156) or usual care (N=155). A secure mobile personal health record (mPHR) app provided participants in the intervention group with key information about diagnoses, medications, and laboratory test values and allowed them to track health goals. The primary study outcome was a chart-derived composite measure of quality of cardiometabolic and preventive services.
Results:
At 12-month follow-up, participants in the mPHR group maintained high quality of care (70% of indicated services at baseline and at 12-month follow-up), in contrast to a decline in quality for the usual-care group (71% at baseline and 67% at follow-up), resulting in a statistically significant but clinically modest differential impact between the groups. No differences between the study groups were found in secondary self-reported outcomes, including delivery of chronic illness care, patient activation, and quality of life related to mental or general medical health.
Conclusions:
Use of a mPHR app was associated with a statistically significant but clinically modest differential benefit for quality of medical care among individuals with serious mental illness and comorbid cardiometabolic conditions.
HIGHLIGHTS
This randomized study tested whether a mobile personal health record (mPHR) application could improve quality of medical care for individuals treated in behavioral health homes that provide onsite primary medical care in mental health clinics.
The study enrolled 311 participants with a serious mental illness and one or more cardiometabolic risk factors across two behavioral health homes.
The mPHR app provided participants in the intervention group with key information about diagnoses, medications, and laboratory test values and allowed them to track health goals.
At 12-month follow up, participants in the intervention group had a statistically significant but clinically modest differential benefit for the intervention relative to the control group.
Patients with serious mental illness are at risk for poor quality of general medical care (1, 2). Challenges in illness self-management (3), stigmatizing attitudes among general medical providers (4), and poor coordination between medical and mental health delivery systems (5) can limit these individuals’ ability to obtain appropriate and timely medical services (2). Poor quality of care, in turn, represents an important risk factor for disability and early mortality in this population (6, 7).
Behavioral health homes, in which routine medical services are provided onsite at a community mental health center, are increasingly being used to improve access and coordination of care in this population (8, 9). However, although these models hold promise for improving quality medical care (10), challenges to their effective implementation remain (11, 12). Limitations to health information technology, including the ability to integrate information across electronic medical and mental health records, remain among the most important barriers to effective implementation of these models (13).
In the general population, use of electronic personal health records (PHRs) has been identified as a promising way to shift the ownership and locus of health records from distribution across multiple providers to an approach that is longitudinal and person centered (14). Still, early efforts to develop Web-based PHRs have been hampered by challenges in interoperability across different platforms and in-patient accessibility, with rates of meaningful use below 10% (15, 16).
The growing use and capability of smartphones provides an opportunity to develop PHRs for mobile platforms (mPHRs) (17). These new technologies offer the potential for ubiquitous access to medical information and, with the adoption of new interoperability standards, the ability to harmonize data across multiple records (18). More than four-fifths of Americans now own smartphones, up from just 35% in 2011, with growing penetration among vulnerable populations, including those with serious mental illnesses (19). The recent introduction of mPHR capability within Apple Health may herald the more widespread use of mPHRs by the general public (20).
The development and implementation of mPHRs may be particularly important for populations with serious mental illness, which have high rates of comorbid conditions and poor coordination between medical and mental health care. However, few data exist assessing the potential effect of these programs among these individuals. Several studies have found that electronic health records are feasible to implement in populations with serious mental illness, either in isolation (21, 22) or as part of a larger set of care manager interventions (23), and hold potential to improve the quality of medical care (24). Reflecting the technologies available at the time, these PHRs were all Web-based and were either tethered to a single electronic health record or required manual data entry.
This randomized trial tested an integrated mPHR designed for patients with serious mental illnesses and comorbid medical conditions treated in behavioral health homes. The goal of the study was to assess the intervention’s impact on quality of medical care and on other clinical outcomes.
Methods
Overview
Recruitment, eligibility, and randomization.
The study was conducted in two behavioral health homes, one in an urban community mental health center and one in a suburban community mental health center. Potential participants were referred by mental health providers or selected from a roster of active patients in the clinics. The first inclusion criterion was the presence of a serious mental illness (schizophrenia, schizoaffective disorder, bipolar disorder, major depression, obsessive-compulsive disorder, or posttraumatic stress disorder, with or without comorbid substance use) (25) confirmed by chart review. The second inclusion criterion was one or more cardiometabolic risk factors (diabetes, hypertension, or hypercholesterolemia) confirmed by chart review. The exclusion criterion was cognitive impairment based on a score of 3 or higher on a six-item, validated screening instrument (26). Participants who met the eligibility criteria and provided informed consent to participate were randomly assigned to either the intervention group or usual care. Assignments were stratified at the patient level within each clinic.
Each behavioral health home provided preventive screening, general medical care, and obstetric/gynecological services onsite at the mental health clinic. One of the clinics had two separate records for mental health and medical care, and the other, which was a part of a larger safety-net health system, had a combined record.
Study arms.
The intervention group received the mPHR app. A total of 64 (41%) of participants in the intervention group did not have smartphones; to ensure representativeness to the broader population, these participants received study smartphones loaded with the mPHR app.
The study app was developed using principles of user-centered design (27), with a particular focus on usability in a medically complex population who commonly have limited health and computer literacy. Results from two focus groups were used to identify key domains for inclusion in the mPHR and to develop and refine a user interface appropriate for the population.
The app was programmed using Sencha Touch in conjunction with the native Java Android HealthVault software development kit, a platform developed by Microsoft to store health information for the PHR. Patient portals from electronic health records were used to upload data to the mPHR; the Fast Healthcare Interoperability Resources standard (18) was used to populate fields on the handheld device from data across the electronic health records and to harmonize data. The HealthVault security system works with cryptographic constructs available more widely on smartphones to preserve user privacy. All information was stored on a server in compliance with the Health Insurance Portability and Accountability Act.
The mPHR included fields for current medications; allergies; anthropomorphic measures, including blood pressure, weight, body mass index, and waist circumference; and medical records information, including a list of conditions, immunizations, and laboratory test values, such as glucose, cholesterol, and hemoglobin A1c. Data were refreshed from the health record whenever a participant used the app. The app also included a field for participants to identify long-term health goals. These goals were broken down into action plans, which involved setting short-term, feasible clinical targets and tracking progress toward those targets (28).
Two trained certified peer specialists subcontracted through the state’s mental health consumer network served as clinical technology specialists (29), providing training and support for participants in the use of the app. The role of the peer specialists was limited to assistance with the use of the smartphone and mPHR app. The training sessions included instruction in use of the app and, as needed, in the use of the mobile phones. Teach-back approaches were used to assess participants’ ability to demonstrate key functions of the smartphone and app (30). Subsequently the peer support specialists met monthly with participants to troubleshoot any issues with the study app or smartphone. They did not provide any care management services, such as scheduling appointments or laboratory tests, and did not assist with data entry.
Each clinical peer specialist received a two-week training program in use of the personal health record from the research staff. The program included a manual providing structured guidance in use of the health record. The research team provided weekly supervision for the clinical peer specialists, reviewing caseloads and troubleshooting any problems that arose.
The usual care group had access to the full array of services offered through the behavioral health homes but did not receive an mPHR app and did not have access to the clinical technology specialists.
Outcomes.
The primary study outcome was a composite measure of quality of preventive medical services and cardiovascular care. Indicators from RAND’s Community Quality Index study (31) were constructed on the basis of reviews of participants’ medical and mental health charts conducted by unblinded research interviewers. These measures have been validated in general populations based on their demonstrated link with improved health outcomes (32). Quality of preventive medical services and eligible populations were derived from the U.S. Preventive Services Task Force guidelines, with 11 indicators used for the study addressing the following conditions: blood pressure, cholesterol, colorectal cancer, diabetes, HIV, obesity, tobacco use, sexually transmitted diseases, breast cancer, cervical cancer, and chlamydia (21). (A list of all quality indicators, along with the eligible populations for each, is available in an online supplement.) Because most of the individual subscales applied only to limited demographic or clinical subsets of the population, a single, aggregate quality score across all conditions was used as the primary study outcome. The aggregate score represents the total number of eligible services received for an individual generated by dividing all instances in which recommended care was delivered by the number of times a participant was eligible for the indicator.
The study also collected secondary self-reported outcome measures. Coordination of care was assessed using the Patient Assessment of Chronic Illness Care measure, a 20-item patient self-report instrument that assesses the extent to which patients with chronic illness report receiving care that aligns with the chronic care model (33–35). Activation, which assesses a patients’ perceived ability to manage their illnesses and their health care visits, was assessed using the Patient Activation Measure (36). Health-related quality of life was measured using the Physical and Mental Component Summary scales of the 12-item Short Form Health Survey (SF-12) (37–39).
Data on app usage were collected for all study participants in the intervention group. A threshold of at least weekly app usage was established a priori as minimum usage representing fidelity to the study intervention. The study protocol was approved by the Emory University Institutional Review Board.
Data Analysis
All analyses were conducted as intent-to-treat analyses using SAS/STAT, version 9.4 (SAS Institute, Cary, NC) and SPSS, version 21.0. Analysis of variance and chi-square tests were used to compare the distribution of baseline characteristics between the intervention and the control group. Marginal linear models and generalized estimating equations were used to analyze outcomes measured over time, and the restricted maximum likelihood and maximum likelihood estimation approaches were used to account for the longitudinal nature of the data and to handle missing data. The SAS PROC GLIMMIX procedure was used for continuous variables, and the PROC GENMOD procedure was used for binary variables. Likelihood ratio tests were conducted to evaluate a meaningful and reasonable symmetric covariance structure for each marginal linear model. For each outcome measure, the model assessed the outcome as a function of randomization, time since randomization, and group-by-time interaction. The group-by-time interaction, which reflects the relative difference in change in the parameters over time, was the primary measure of statistical significance.
To assess whether there was a differential effect by study site or baseline patient characteristics, moderator analyses were conducted for clinic site, cardiometabolic disease morbidity (one versus two or more cardiometabolic conditions), and smartphone ownership status at the time of enrollment. Analyses examined the interaction of group, time, and moderator across each of these categories.
To account for possible loss to follow-up or missing responses, multiple imputation procedures were performed as sensitivity analyses. Little’s missing completely at random (MCAR) test was used to examine the missing mechanism assumption for multiple imputation, and there was no evidence suggesting that the missing data were not missing completely at random (Little’s test p=0.73) (40). The multiple imputation model included all key analysis variables, variables that were correlated with the analysis variables, and variables that might predict missing data on the analytic variables. Twenty data sets were imputed using a fully conditional specification method. The variability between the estimates from the 20 imputations and those from the main analysis were minimal because missing data were missing completely at random.
Hypotheses were two sided and tested at a 0.05 significance level. We prespecified a single primary outcome (composite quality of medical care) to minimize type I error and used a p value of 0.05 for exploratory analyses of secondary outcomes in order to minimize the potential for type II error (41–43).
Results
Of 458 participants screened for eligibility, 311 were eligible and consented to participate (see online supplement). The most common reason for lack of eligibility was not meeting the inclusion criterion of having a cardiometabolic condition. Participants were randomly assigned to either the intervention group (N=156) or the usual-care group (N=155).
Chart review data, which were used to assess overall quality of care, were available for all participants at baseline and 12-month follow-up. Completion rates for patient interviews were 92% at 6 months and 85% at 12 months, with similar rates of attrition in both groups (see online supplement).
The mean age of participants was 50.66±8.76 years, 60% (N=187) of participants were female, 77% (N=311) were African American, and most were either uninsured (N=77, 50%) or covered by Medicaid (N=109, 35%). The most common mental health diagnosis was major depression (N=238, 77%), and the most common cardiometabolic comorbid conditions were hypertension (N=257, 82%) and diabetes (N=136, 44%). None of the demographic or clinical characteristics differed significantly between the intervention and control groups (Table 1).
| Mobile personal health record app (N=156) | Usual care (N=155) | ||||
|---|---|---|---|---|---|
| Characteristic | N | % | N | % | p |
| Age (M±SD years) | 50.78±8.45 | 50.54±9.08 | .81 | ||
| Male | 61 | 39 | 63 | 41 | .78 |
| Single | 94 | 60 | 82 | 53 | .19 |
| Race | .21 | ||||
| White | 29 | 19 | 29 | 19 | |
| African American | 118 | 76 | 123 | 79 | |
| Other race | 9 | 6 | 3 | 2 | |
| Hispanic | 2 | 1 | 4 | 3 | .41 |
| Education (M±SD years) | 12.15±2.30 | 12.11±2.36 | .87 | ||
| Stable housing | 124 | 79 | 136 | 88 | .05 |
| Total annual income | .99 | ||||
| ≤$5,000 | 86 | 55 | 83 | 54 | |
| $5,000–$10,000 | 42 | 27 | 43 | 28 | |
| $10,000–$15,000 | 18 | 12 | 17 | 11 | |
| >$15,000 | 8 | 5 | 8 | 5 | |
| Stable employment | 18 | 12 | 12 | 8 | .26 |
| Disability | 63 | 40 | 63 | 41 | .96 |
| Medicaid | 57 | 37 | 52 | 34 | .58 |
| Medicare | 28 | 18 | 35 | 23 | .31 |
| Uninsured | 77 | 49 | 80 | 52 | .69 |
| Private insurance | 24 | 15 | 35 | 23 | .17 |
| Medical diagnosis | |||||
| Diabetes | 68 | 44 | 68 | 44 | .83 |
| Heart disease, coronary artery disease, coronary heart disease | 13 | 8 | 13 | 8 | .96 |
| Hyperlipidemia | 72 | 46 | 70 | 45 | .86 |
| Hypertension | 134 | 86 | 123 | 79 | .35 |
| Mental disorder diagnosis | |||||
| Schizophrenia | 34 | 22 | 33 | 21 | .91 |
| Bipolar disorder | 43 | 28 | 41 | 26 | .63 |
| Depression | 116 | 74 | 122 | 79 | .24 |
| Obsessive-compulsive disorder | 4 | 3 | 6 | 4 | .54 |
| Posttraumatic stress disorder | 35 | 22 | 26 | 17 | .20 |
TABLE 1. Baseline characteristics of participants with serious mental illness and comorbid cardiometabolic conditions who received a mobile personal health record smartphone application or usual care
Within the intervention group, 70% (N=156) of participants used the app at least once per week, the minimum prespecified for fidelity to the intervention (Figure 1). Most app events involved viewing, with a smaller percentage editing or adding items (see online supplement).
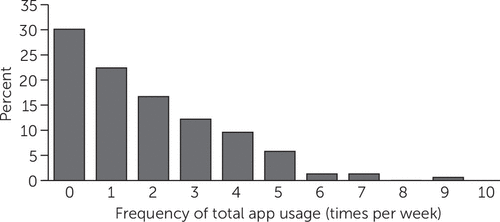
FIGURE 1. Frequency of use of a mobile personal health record smartphone application among 156 study participants with serious mental illness and comorbid cardiometabolic conditionsa
aMedian usage=1 time/week; mean usage=1.8 times/week.
The primary study outcome was receipt of indicated cardiometabolic and preventive services. Participants in the intervention group received 70% of indicated services on the outcome measure at baseline and at 12-month follow-up, compared with a decline from 71% to 67% in the usual-care group. The group-by-time effect for this interaction was statistically significant (F=4.18; df=1, 309; p=0.04) (Table 2).
| Mobile personal health record (N=156) | Usual care (N=155) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Mean | SD | N | Mean | SD | F | df | pa |
| Patient Assessment of Chronic Illness Careb | .61 | 2, 854 | .54 | ||||||
| Baseline | 156 | 3.20 | .95 | 155 | 3.23 | 1.00 | |||
| 6 months | 145 | 3.32 | .93 | 141 | 3.32 | 1.01 | |||
| 12 months | 132 | 3.25 | .94 | 131 | 3.18 | .95 | |||
| Patient Activation Measurec | .71 | 2, 855 | .49 | ||||||
| Baseline | 156 | 58.78 | 14.65 | 155 | 57.83 | 13.65 | |||
| 6 months | 145 | 59.82 | 15.89 | 141 | 60.88 | 15.18 | |||
| 12 months | 132 | 61.61 | 16.19 | 132 | 62.08 | 14.77 | |||
| SF-12 Physical Component Summaryd | .32 | 2, 821 | .73 | ||||||
| Baseline | 147 | 35.74 | 11.83 | 150 | 35.70 | 11.62 | |||
| 6 months | 138 | 37.20 | 11.94 | 133 | 36.32 | 11.52 | |||
| 12 months | 129 | 37.03 | 12.15 | 130 | 36.09 | 11.75 | |||
| SF-12 Mental Component Summaryd | .56 | 2, 821 | .57 | ||||||
| Baseline | 147 | 35.50 | 12.54 | 150 | 36.55 | 13.25 | |||
| 6 months | 138 | 39.16 | 13.48 | 133 | 38.70 | 13.18 | |||
| 12 months | 129 | 41.68 | 14.04 | 130 | 41.16 | 11.61 | |||
| Body mass index (kg/m2) | .16 | 2, 851 | .86 | ||||||
| Baseline | 155 | 33.00 | 8.18 | 155 | 33.37 | 8.18 | |||
| 6 months | 145 | 32.87 | 7.74 | 139 | 33.71 | 8.31 | |||
| 12 months | 132 | 32.49 | 7.78 | 131 | 33.27 | 8.46 | |||
| Smokinge | 5.65 | 2 | .06 | ||||||
| Baseline | 70 | 45 | — | 60 | 39 | — | |||
| 6 months | 65 | 45 | — | 49 | 35 | — | |||
| 12 months | 49 | 37 | — | 46 | 35 | — | |||
| Composite quality of preventive medical services and cardiovascular caref | 4.18 | 1, 309 | .04 | ||||||
| Baseline | 156 | .70 | .11 | 155 | .71 | .12 | |||
| 12 months | 156 | .70 | .15 | 155 | .67 | .16 | |||
TABLE 2. Outcome measures among participants with serious mental illness and comorbid cardiometabolic conditions who received a mobile personal health record smartphone application or usual care
Moderator analyses were conducted to assess whether this outcome differed significantly by site or clinical characteristics. The moderator-by-group-by-time variables were not significant for study site, cardiometabolic comorbid condition, or smartphone ownership over time, suggesting that none of these variables moderated the main study effect (see online supplement).
There were no clinically significant group-by-time interactions for the intervention versus usual care on self-reported coordination of chronic illness care, patient activation, or quality of life related to mental health or physical health (Table 2). Scores for the Patient Activation Measure and mental component summary of the SF-12 showed statistically significant improvement in both the intervention group (baseline vs. 12-month: 58.78±14.65 vs. 61.61±16.19, t=2.46, df=855, p=0.01; baseline vs. 12-month: 35.50±12.54 vs. 41.68±14.04, t=5.28, df=821, p<0.01, respectively) and the control group (baseline vs. 12-month: 57.83±13.65 vs. 62.08±14.77, t=3.52, df=855, p<0.01; baseline vs. 12-month: 36.55±13.25 vs. 41.16±11.61, t=3.96, df=821, p<0.01, respectively).
Discussion
The study found that a mMPH app was associated with a statistically significant but clinically modest differential benefit for quality of medical care among individuals with serious mental illness and comorbid cardiometabolic conditions. There were no changes in self-reported outcomes, including delivery of chronic illness care, patient activation, or quality of life related to mental health or physical health.
More than two-thirds of participants in the intervention group used the mobile app at least weekly. Promoting regular use of personal health records has been challenging, with low rates of uptake attributed to cumbersome data entry and availability of the records (44). In the current study, rates of use were relatively high, although this usage largely involved viewing results rather than actively entering or editing data such as health goals. Future research, including qualitative studies, should continue to delineate the barriers to meaningful use of these technologies in populations with serious mental illness and develop ways to overcome these barriers. Research should also examine the best roles for PHRs, which can support but are also dependent on patient engagement, versus approaches at the level of the health system for integration of medical and psychiatric health information.
One feature in the current study that may have facilitated participants’ use of the PHR is the role of peer specialists in engaging participants in using the intervention. Clinical technology specialists, who train and support patients in the use of health information technology interventions, have been proposed as a strategy for promoting uptake and ongoing use of mobile health interventions (29). Peer specialists have particular expertise in supporting engagement in interventions and fostering positive health behavior change (45). With appropriate training, these providers may be able to play a useful role in supporting implementation of mobile health interventions in populations with serious mental illness (46).
The quality of medical services in the behavioral health homes was substantially higher than seen in usual care for community mental health centers (47) and the general population (31). These findings provide evidence supporting the potential for behavioral health homes to deliver high-quality medical services to their patients with mental illness (10). However, for the current study, it may also have created a ceiling effect that limited the ability to detect an effect of the intervention on quality of care, compared with mental health settings that do not have integrated medical services (24).
Other secondary outcomes, including health-related quality of life and cardiometabolic risk, did not differ significantly between the intervention group and the usual-care group. The study’s findings support the observation from general populations that while mobile health interventions may be important facilitators of change for patients, their use in isolation may have a more limited effect on distal health improvements (48).
The findings should be interpreted in the light of several limitations. First, the project did not include a formal qualitative aim, limiting our ability to assess participants’ perspectives on the app and how it affected their service use. Second, although structured data collection forms were used to mitigate against potential bias (49), it was not feasible for the reviewers for the chart review or patient interviews to be fully blinded to the participants’ group. Finally, the pragmatic design and broad inclusion criteria resulted in heterogeneity across patient and clinic characteristics and may have reduced the ability to detect a larger effect of the mobile PHR (50).
Conclusions
With the growing use of smartphones and patient portals for electronic health records, patients, including those with serious mental illness, will increasingly have easy access to their health records. The study’s findings suggest that, particularly when used in integrated settings, these interventions may have limited incremental value. More work is needed to understand how they can be incorporated into broader efforts to improve quality and outcomes of medical care in this population.
1 : Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry 2009; 194:491–499Crossref, Medline, Google Scholar
2 : Quality of medical care for persons with serious mental illness: a comprehensive review. Schizophr Res 2015; 165:227–235Crossref, Medline, Google Scholar
3 : Chronic disease self-management interventions for adults with serious mental illness: a systematic review of the literature. Gen Hosp Psychiatry 2014; 36:233–244Crossref, Medline, Google Scholar
4 : General medical clinicians’ attitudes toward people with serious mental illness: a scoping review. J Behav Health Serv Res 2019; 46:656–679Crossref, Medline, Google Scholar
5 : From silos to bridges: meeting the general health care needs of adults with severe mental illnesses. Health Aff 2006; 25:659–669Crossref, Medline, Google Scholar
6 : Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol 2010; 24(Suppl):69–80Crossref, Medline, Google Scholar
7 : Association of secondary preventive cardiovascular treatment after myocardial infarction with mortality among patients with schizophrenia. JAMA Psychiatry 2018; 75:1234–1240Crossref, Medline, Google Scholar
8 : Specialty care medical homes for people with severe, persistent mental disorders. Health Aff 2010; 29:867–873Crossref, Medline, Google Scholar
9 : Integrating primary care into community behavioral health settings: programs and early implementation experiences. Psychiatr Serv 2013; 64:660–665Link, Google Scholar
10 : Randomized trial of an integrated behavioral health home: the Health Outcomes Management and Evaluation (HOME) study. Am J Psychiatry 2017; 174:246–255Link, Google Scholar
11 : Physical health outcomes and implementation of behavioural health homes: a comprehensive review. Int Rev Psychiatry 2018; 30:224–241Crossref, Medline, Google Scholar
12 : Systematic review of the impact of behavioral health homes on cardiometabolic risk factors for adults with serious mental illness. Psychiatr Serv 2020; 71:57–74Link, Google Scholar
13 Scharf D, Vaughan C, Eberhart N, et al: Integrating Primary Care Services Into Behavioral Health Settings for Persons With Serious Mental Illness: First Lessons Learned From SAMHSA’s Primary and Behavioral Health Care Integration (PBHCI) Grants Program. Presented at the 2011 SAMHSA PBHCI Grantee Meeting, San Diego, May 4–5, 2011Google Scholar
14 : Personal health records: a systematic literature review. J Med Internet Res 2017; 19:
15 Markle Survey: PHR Adoption on the Rise; 1 in 10 Say They Have Electronic PHR. New York, Markle Foundation, 2011. http://www.markle.org/sites/default/files/5_PHRs.pdfGoogle Scholar
16 : Factors that affect the use of electronic personal health records among patients: a systematic review. Int J Med Inform 2019; 126:164–175Crossref, Medline, Google Scholar
17 : Smart health: concepts and status of ubiquitous health with smartphone; in Proceedings of the International Conference on Information and Communication Technology Convergence (ICTC). Piscataway, NJ, IEEE, 2011Google Scholar
18 : HL7 FHIR: an Agile and RESTful approach to healthcare information exchange; in Proceedings of the 26th IEEE International Symposium on Computer-Based Medical Systems. Piscataway, NJ, IEEE, 2013Google Scholar
19 : Smartphone ownership, use, and willingness to use smartphones to provide peer-delivered services: results from a national online survey. Psychiatr Q 2018; 89:947–956Crossref, Medline, Google Scholar
20 : Personal health records: more promising in the smartphone era? JAMA 2019; 321:339–340Crossref, Medline, Google Scholar
21 : Electronic personal health records for people with severe mental illness: a feasibility study. BMC Psychiatry 2015; 15:192Crossref, Medline, Google Scholar
22 : Collaborative development of an electronic personal health record for people with severe and enduring mental health problems. BMC Psychiatry 2014; 14:305Crossref, Medline, Google Scholar
23 : Using electronic health records to enhance a peer health navigator intervention: a randomized pilot test for individuals with serious mental illness and housing instability. Community Ment Health J 2018; 54:1172–1179Crossref, Medline, Google Scholar
24 : Randomized trial of an electronic personal health record for patients with serious mental illnesses. Am J Psychiatry 2014; 171:360–368Link, Google Scholar
25 : An empirical literature review of definitions of severe and persistent mental illness. Am J Psychiatry 1990; 147:1602–1608Link, Google Scholar
26 : Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002; 40:771–781Crossref, Medline, Google Scholar
27 : A user-centered model for designing consumer mobile health (mHealth) applications (apps). J Biomed Inform 2016; 60:243–251Crossref, Medline, Google Scholar
28 : Action planning: a call to action. J Am Board Fam Med 2006; 19:324–325Crossref, Medline, Google Scholar
29 : Clinical technology specialists. BMJ 2015; 350: h945Google Scholar
30 : The effectiveness of the teach-back method on adherence and self-management in health education for people with chronic disease: a systematic review. JBI Database Syst Rev Implement Reports 2016; 14:210–247Crossref, Medline, Google Scholar
31 : The quality of health care delivered to adults in the United States. N Engl J Med 2003; 348:2635–2645Crossref, Medline, Google Scholar
32 : The reproducibility of a method to identify the overuse and underuse of medical procedures. N Engl J Med 1998; 338:1888–1895Crossref, Medline, Google Scholar
33 : Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care 2005; 43:436–444Crossref, Medline, Google Scholar
34 : Use of the Patient Assessment of Chronic Illness Care (PACIC) with diabetic patients: relationship to patient characteristics, receipt of care, and self-management. Diabetes Care 2005; 28:2655–2661Crossref, Medline, Google Scholar
35 : A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care 2005; 11:478–488Medline, Google Scholar
36 : Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004; 39:1005–1026Crossref, Medline, Google Scholar
37 : Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998; 51:1171–1178Crossref, Medline, Google Scholar
38 : A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med 1997; 19:179–186Crossref, Medline, Google Scholar
39 : Reliability and validity of the SF-12 health survey among people with severe mental illness. Med Care 2000; 38:1141–1150Crossref, Medline, Google Scholar
40 : A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 1988; 83:1198–1202Crossref, Google Scholar
41 : No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43–46Crossref, Medline, Google Scholar
42 : Multiplicity in randomised trials I: endpoints and treatments. Lancet 2005; 365:1591–1595Crossref, Medline, Google Scholar
43 : Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2002; 2:8Crossref, Medline, Google Scholar
44 : Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J Am Med Inform Assoc 2006; 13:121–126Crossref, Medline, Google Scholar
45 : Integrating wellness, recovery, and self-management for mental health consumers. Community Ment Health J 2010; 46:130–138Crossref, Medline, Google Scholar
46 : Peer support: a human factor to enhance engagement in digital health behavior change interventions. Journal of Technology in Behavioral Science 2019; 4:152–161Crossref, Medline, Google Scholar
47 : A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry 2010; 167:151–159Link, Google Scholar
48 : Wearable devices as facilitators, not drivers, of health behavior change. JAMA 2015; 313:459–460Crossref, Medline, Google Scholar
49 : Pragmatic randomized clinical trials: best practices and statistical guidance. Health Serv Outcomes Res Methodol 2019; 19:23–35Crossref, Google Scholar
50 : Moderators of treatment outcomes: clinical, research, and policy importance. JAMA 2006; 296:1286–1289Crossref, Medline, Google Scholar



