Lung Cancer Screening Eligibility, Risk Perceptions, and Clinician Delivery of Tobacco Cessation Among Patients With Schizophrenia
Abstract
Objective:
Individuals with schizophrenia experience increased lung cancer mortality and decreased access to cancer screening and tobacco cessation treatment. To promote screening among individuals with schizophrenia, it is necessary to investigate the proportion who meet screening criteria and examine smoking behaviors, cancer risk perception, and receipt of tobacco cessation interventions from psychiatry and primary care.
Methods:
The authors performed a cross-sectional survey and medical record review with 112 adults with schizophrenia treated with clozapine in a community mental health clinic (CMHC).
Results:
Among older participants (ages 55–77 years) with schizophrenia, 34% met the criteria for lung screening on the basis of smoking history (heavy current or former smokers), and more than half believed they had a low risk of developing lung cancer. Of all participants, 88% had visited their primary care provider (PCP) in the past year; PCPs represented 35 different practices. Only one in three current smokers reported that their PCP or psychiatrist assisted them in obtaining medications for tobacco cessation.
Conclusions:
Given smoking history, many older adults with schizophrenia have potential to benefit from lung screening, yet most older participants underestimated their lung cancer risk. Although participants regularly accessed care, PCP and psychiatric visits may be missed opportunities to engage patients with schizophrenia in tobacco cessation and decrease preventable premature mortality. Embedding interventions in a CMHC, a centralized access point of care delivery for patients with schizophrenia, may have unique potential to increase uptake of cancer screening and tobacco cessation interventions.
HIGHLIGHTS
Among older participants (ages 55–77 years) with schizophrenia, 34% met criteria for lung cancer screening on the basis of smoking history (heavy current or former smokers).
More than half of current and former smokers believed they had a low risk of developing lung cancer.
Among all current smokers, only one in three reported that their primary care provider (PCP) or psychiatrist assisted with their obtaining medications for tobacco cessation.
Although participants regularly accessed care, PCP and psychiatric visits may be missed opportunities to engage patients with schizophrenia in tobacco cessation treatment and decrease preventable premature mortality.
Adults with schizophrenia are dying 15–25 years earlier than the general population (1–3). This mortality gap is widening (2, 4, 5), and more than two-thirds of the excess deaths are due to smoking-related diseases such as lung cancer (6). Specifically, adults with schizophrenia are more than twice as likely to die from lung cancer than adults in the general population, in part because of delays in cancer diagnosis and inequities in cancer treatment (2, 7, 8). To improve lung cancer outcomes, adults with schizophrenia need to be engaged in tobacco cessation treatment and engaged in interventions designed to promote early cancer detection.
The National Lung Screening Trial (9) demonstrated that lung cancer screening with low-dose computed tomography decreased lung cancer mortality by 21% among high-risk eligible patients when combined with tobacco cessation. The U.S. Preventive Services Task Force (10) subsequently recommended annual screening, and the Centers for Medicare and Medicaid Services (CMS; 11) authorized screening counseling and shared decision making for eligible beneficiaries. However, despite these recommendations, lung cancer screening rates in 2015 remained lower than 4%, with rates among vulnerable patients likely considerably lower (12).
Although the prevalence of smoking in the United States has decreased from 42% to 19% since the 1960s, 60%−80% of adults with schizophrenia currently smoke (13). This population smokes more cigarettes and inhales more deeply, both of which are factors associated with increased risk of lung cancer (14). Moreover, despite clinical practice guidelines recommending the 5As framework (Ask, Advise, Assess, Assist, and Arrange Follow-Up) for tobacco cessation for all patients (15), many mental health professionals are reluctant to address smoking and provide cessation treatment (16). However, adults with schizophrenia can quit safely, particularly with bupropion or varenicline, and especially in conjunction with cessation counseling (17, 18).
Targeted approaches are needed to decrease disparities in lung cancer risk and detection among individuals with schizophrenia. Mental health clinicians may be uniquely suited to address tobacco cessation and educate patients about cancer risk, given established relationships and frequent communication (19). Although primary care providers (PCPs) are more likely than psychiatrists to report participation in patients’ tobacco cessation (20), clinician differences in delivery of tobacco cessation to adults with serious mental illness remains unstudied. In addition, although data are lacking regarding rates of lung cancer screening for patients with schizophrenia, we expect lower rates given disparities in rates of breast (6%−20% lower) and colorectal (>20% lower) cancer screening compared with patients without schizophrenia (21, 22). Thus, in this study we aimed to establish the prevalence of older adults with schizophrenia who meet eligibility criteria for lung cancer screening, investigate patient lung cancer risk perceptions, and examine patient-reported PCP access and clinician delivery of smoking cessation interventions.
Methods
Study Population and Design
In 2014, we conducted a cross-sectional study of patients with schizophrenia who were receiving treatment with clozapine at a community mental health clinic (CMHC). Eligible participants were 18 years or older, diagnosed as having a schizophrenia spectrum disorder (schizophrenia or schizoaffective disorder), and treated in a clozapine clinic and had scheduled visits at the CMHC at least every four weeks. Mental health clinicians assessed whether potentially eligible patients had the capacity to be approached to participate in the study. Reasons for exclusion included severe disorganization or agitation that affected the ability to complete the questionnaire.
A member of the research team obtained consent and administered the survey during a mental health appointment. Participants provided consent to access their CMHC medical records and request records from their PCP. They received a $15 gift card for their time. Study procedures were approved by the Partners Healthcare Institutional Review Board.
Measures
Participants completed a survey that included sociodemographic questions (age, gender, ethnicity, race, education), the Heaviness of Smoking Index (23), smoking behaviors, patient cancer risk perceptions, and clinician delivery of tobacco cessation through the 5As framework (15). All questionnaire items were validated with a national sample during the National Lung Screening Trial (9). Board-certified psychiatrists and psychologists with expertise in psychotic disorders and cancer risk perceptions reviewed the measures for burden and fit for patients with schizophrenia.
Eligibility for screening was based on CMS guidelines (age 55–77 years, current or former smokers within the past 15 years, ≥30 packs per year smoking history). We also assessed the number of current smokers ages 40–54 who would become eligible for screening at age 55 if they continued to smoke. Sensitivity analyses were conducted for the subgroup of current and former smokers who met age criteria for screening (age ≥55 years) regarding their cancer risk perceptions compared with those of the overall group.
Smoking Behaviors
Nicotine dependence was determined on the basis of the Heaviness of Smoking Index (23). Additional items from the National Lung Screening Trial questionnaire included readiness to quit smoking (ranging from “I enjoy smoking so much and will never consider quitting” to “I have quit and am 100% confident I will never smoke again”) and quit attempts within the past year.
Lung Cancer Risk Perceptions
For current and former smokers, we assessed National Lung Screening Trial items on cancer risk perceptions, including the participants’ beliefs about their risk of developing lung cancer (five items, scored on a scale ranging from very high to very low), whether the patient is in danger of developing lung cancer because they smoke or used to smoke (five items, scored on a scale ranging from strongly agree to strongly disagree), perceived risk of developing lung cancer compared with other smokers or former smokers (five items, scored on a scale ranging from much lower to much higher), how much would or did quitting reduce their risk of developing lung cancer (four items, scored on a scale ranging from not at all to very much), how likely the patient is to develop lung cancer compared with the average person their age (three items, scored on a scale ranging from less likely to more likely), the additional risk of lung cancer for a pack-a-day smoker compared with a nonsmoker (no additional risk, two times, five times, 10 times, and 20 times), and whether the patient worries about developing lung cancer (four items, scored on a scale ranging from rarely or never to all the time). Participants were classified as underestimating risk if they reported that the additional risk of lung cancer from smoking one pack per day was “no additional risk” or “two times the risk.”
Clinician Delivery of Tobacco Cessation
As in the National Lung Screening Trial, we asked current smokers about clinician delivery of the 5As framework for tobacco cessation. We compared patient-reported interactions with primary care and psychiatry for delivery of the 5As.
Data Analysis
We conducted data analysis using SAS, version 9.4 (24). Descriptive statistics characterized demographic characteristics, smoking behaviors, clinician-delivered tobacco cessation, and lung cancer risk perceptions. Comparisons of dichotomized variables were performed by using Fisher’s exact test. Dichotomized variables were as follows: “How high do you think your risk of developing lung cancer is?” (very or somewhat low versus moderate, somewhat, or very high); “I am in danger of developing lung cancer because I smoke or used to smoke” (disagree or strongly disagree versus neutral, agree, or strongly agree); “Compared with other current or former smokers, what is your risk of getting lung cancer?” (much or slightly lower versus about the same, slightly, or much higher); “How much would or did quitting reduce your chances of developing lung cancer?” (not at all versus a little, somewhat, or very much); “[What is] your likelihood of developing lung cancer compared with same-aged person?” (less likely versus about as likely or more likely); “How much higher is the risk of developing lung cancer for a pack-per-day smoker than a nonsmoker? (no additional risk or two times the risk versus five or 20 times the risk or doesn’t know); “How often do you worry about developing lung cancer?” (rarely or never versus sometimes, often, or all the time); and the Heaviness of Smoking Index (moderate or high dependence versus low dependence).
We calculated whether participants were motivated to quit by grouping responses to the items “I plan to quit in the next 6 months,” “I plan to quit in the next 30 days,” and “I have already cut down and have set a quit date.” We calculated whether participants thought about quitting but had no active plans to quit by grouping responses to “I [rarely, sometimes, or often] think about quitting and have no plans to quit.” We calculated pack-years by dividing the average number of packs smoked per day for each participant by years of smoking history.
Results
Patient Characteristics
At the clozapine clinic, we requested clinicians’ permission to approach 199 patients who met the eligibility criteria; 22 were then excluded because of paranoia or disorganization that impaired study participation, and 26 were not available for approach in the clinic. Study staff approached 151 patients, and 113 (75%) consented to participate. One participant withdrew (N=112).
Sociodemographic Characteristics
Sociodemographic characteristics are displayed in Table 1. Participants were 71% male and had a mean age of 44.9 years. Thirty-two patients (29%) were 55 years or older, and 37 (33%) were 40–54 years old. Seventy-one percent were white, 24% had a college education, and 19% did not complete high school or receive a GED.
| Characteristic | N | % |
|---|---|---|
| Age (M±SD) | 44.9±13.5 | |
| Gender | ||
| Male | 79 | 71 |
| Female | 33 | 30 |
| Race-ethnicity | ||
| Hispanic or Latino | 9 | 8 |
| Non-Hispanic or Latino | 98 | 92 |
| White | 77 | 71 |
| Black or African American | 15 | 14 |
| American Indian or Alaska Native | 2 | 2 |
| Asian | 2 | 2 |
| Other | 12 | 11 |
| Highest education level | ||
| Did not complete high school | 20 | 19 |
| Completed high school or GED | 20 | 19 |
| Some college | 41 | 38 |
| Completed college or higher | 26 | 24 |
| Perceived overall health | ||
| Excellent condition | 12 | 11 |
| Very good or good condition | 75 | 67 |
| Fair condition | 21 | 19 |
| Poor condition | 4 | 4 |
| Primary care utilization | ||
| Identified primary care provider (PCP) | 105 | 94 |
| Saw PCP in past year | 99 | 88 |
| Tobacco use | ||
| Current smoker | 50 | 45 |
| Former smoker | 38 | 34 |
| Never smoker | 24 | 21 |
TABLE 1. Characteristics of 112 CMHC patients with schizophreniaa
Screening Eligibility
Of the participants, 79% were current or former tobacco smokers (45% and 34%, respectively); 21% were never smokers (Table 1). Among older adults (≥55 years), 91% (N=29) were current or former tobacco smokers (44% [N=14] and 47% [N=15], respectively), and 34% (N=11) met CMS screening criteria. Among adults ages 40–54 (current smokers, N=18, 49%), 78% (N=14) of the current smokers would become eligible for screening as they age if they continued to smoke.
Smoking Behaviors
Current cigarette smokers smoked a mean of 17.6 cigarettes per day and had an average smoking history of 24.5 pack-years. Among current smokers, 62% had moderate or high nicotine dependence, and 43% reported at least one quit attempt in the past year. Former smokers smoked a mean of 21.4 cigarettes per day and averaged 26.2 pack-years, and 59% had moderate to high nicotine dependence (Table 2). Of current smokers, 40% (N=18) reported planning to quit and 38% (N=17) reported thinking about quitting but had no active plans to do so (Figure 1); 19% of former smokers reported worrying about relapsing.
| Total (N=88) | Current smokers (N=50) | Former smokers (N=38) | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | p |
| Lung cancer risk perception | |||||||
| How high do you think your risk of developing lung cancer is?b | .008 | ||||||
| Very to somewhat high | 10 | 12 | 9 | 19 | 1 | 3 | |
| Moderate | 25 | 30 | 17 | 35 | 8 | 22 | |
| Very to somewhat low | 49 | 58 | 22 | 46 | 27 | 75 | |
| I am in danger of developing lung cancer because I smoke or used to smokec | .002 | ||||||
| Strongly agree or agree | 31 | 37 | 27 | 55 | 4 | 11 | |
| Neutral | 18 | 21 | 9 | 18 | 9 | 26 | |
| Strongly disagree or disagree | 35 | 42 | 13 | 27 | 22 | 63 | |
| Compared with other current or former smokers, what is your risk of getting lung cancer?d | .002 | ||||||
| Much or slightly higher | 10 | 12 | 9 | 19 | 1 | 3 | |
| About the same | 30 | 36 | 21 | 45 | 9 | 25 | |
| Much or slightly lower | 43 | 52 | 17 | 36 | 26 | 72 | |
| How much would or did quitting reduce your chances of developing lung cancer?e | .289 | ||||||
| Very much | 47 | 56 | 20 | 42 | 27 | 75 | |
| Somewhat | 18 | 21 | 14 | 29 | 4 | 11 | |
| A little | 10 | 12 | 7 | 15 | 3 | 8 | |
| Not at all | 9 | 11 | 7 | 15 | 2 | 6 | |
| [What is] your likelihood of developing lung cancer compared with same-aged person?f | .028 | ||||||
| Less likely to get lung cancer | 36 | 43 | 15 | 32 | 21 | 57 | |
| About as likely to get lung cancer | 37 | 44 | 23 | 49 | 14 | 38 | |
| More likely to get lung cancer | 11 | 13 | 9 | 19 | 2 | 5 | |
| How much higher is the risk of developing lung cancer for a pack-per-day smoker than a nonsmoker?g | .010 | ||||||
| No additional risk | 7 | 9 | 6 | 13 | 1 | 3 | |
| 2× | 20 | 24 | 15 | 32 | 5 | 14 | |
| 5× | 18 | 22 | 7 | 15 | 11 | 31 | |
| 10× | 24 | 29 | 12 | 25 | 12 | 35 | |
| 20× | 10 | 12 | 5 | 11 | 5 | 14 | |
| Doesn’t know | 3 | 4 | 2 | 4 | 1 | 3 | |
| How often do you worry about getting lung cancer?h | .052 | ||||||
| Rarely or never | 47 | 55 | 22 | 46 | 25 | 67 | |
| Sometimes | 21 | 25 | 11 | 23 | 10 | 27 | |
| Often | 11 | 13 | 10 | 21 | 1 | 3 | |
| All the time | 6 | 7 | 5 | 10 | 1 | 3 | |
| Smoking behavior | |||||||
| Cigarettes per day (M±SD) | 17.6±12.1 | 21.4±17.6 | |||||
| Cigarette pack-years (M±SD) | 24.5±18.5 | 26.2±27.2 | |||||
| Heaviness of Smoking Indexi | .820 | ||||||
| Low dependence | 32 | 39 | 18 | 38 | 14 | 41 | |
| Moderate dependence | 37 | 45 | 25 | 52 | 12 | 35 | |
| High dependence | 13 | 16 | 5 | 10 | 8 | 24 | |
TABLE 2. Perception of lung cancer risk and smoking behaviors among 88 CMHC patients with schizophrenia who reported being current or former smokersa
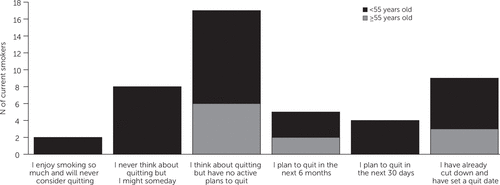
FIGURE 1. Readiness to quit smoking among 45 current smokers with schizophrenia, by age groupa
aPatients ages ≥55 years, N=11; ages <55 years, N=34. Data were missing for five patients.
Older current cigarette smokers smoked a mean of 15.7±10.2 cigarettes per day and had an average smoking history of 32.7±22.4 pack-years. Of older smokers, 75% had moderate to high nicotine dependence, and 42% reported at least one quit attempt in the past year. A larger proportion of older current smokers (six of 11) than younger current smokers (11 of 34) thought about quitting (Figure 1).
Lung Cancer Risk Perceptions
Current and former smokers reported significantly different perceptions of their cancer risk (Table 2). Compared with current smokers, former smokers perceived lower cancer risk; 75% of former smokers believed they had a very or somewhat low risk of developing lung cancer compared with 46% of current smokers (p=0.008). When comparing themselves with other current or former smokers, former smokers were more likely to report having slightly or much lower risk of lung cancer (former smokers, 72%; current smokers, 36%; p=0.002). Former smokers were also less likely than current smokers to report that they were in danger of developing lung cancer (former smokers, 27%; current smokers, 63%; p=0.002) In contrast, current smokers were more likely than former smokers to underestimate the risk of developing lung cancer for a pack-a-day smoker (current smokers, 45%, former smokers, 17%; p=0.010). In addition, 15% of current smokers believed that quitting would not reduce their risk of developing lung cancer.
Although older (>55 years) and younger adults with schizophrenia perceived lung cancer risk similarly, 69% (N=20) of older current and former smokers reported rarely or never worrying about developing lung cancer compared with 55% (N=48) of the overall sample of current and former smokers.
Primary Care Delivery
Of the participants, 94% identified a PCP, and 88% reported at least one PCP visit within the past year (Table 1). All participants received psychiatric care at the CMHC and had monthly psychiatry appointments in the clozapine clinic. Yet, despite being seen in one CMHC, participants received primary care from 91 different PCPs based in 35 distinct primary care practices.
Clinician Delivery of Clinical Practice Guidelines for Tobacco Cessation
Participants who identified as current smokers described delivery of the 5As over the past year by their PCPs and psychiatrists (Figure 2). Although 85% (N=38) reported being asked whether they smoked by their PCP (60% [N=27] by their psychiatrist), only 54% (N=24) reported being advised to quit (45% [N=20] by their psychiatrist). Notably, only one in three current smokers reported being assisted with medications for tobacco cessation by both their PCP (33%, N=15) and their psychiatrist (36%, N=16), and only 13% (N=6) reported being referred for smoking cessation counseling by their PCP (versus 20% [N=9] by their psychiatrist). A similar pattern was observed for older participants (≥55 years).
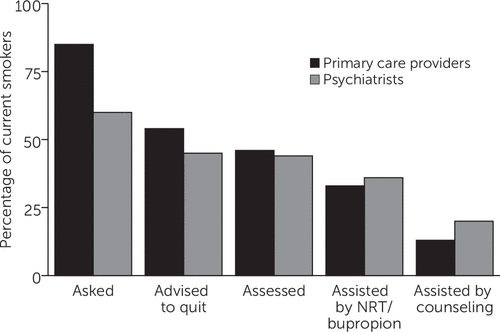
FIGURE 2. Smoking cessation interventions reported by 50 current smokers in the past year, by providera
aInformation was provided by 46 participants for interventions by primary care providers and by 50 participants for interventions by psychiatrists. NRT, nicotine replacement therapy.
Discussion
More than 90% of older adults with schizophrenia seen in a CMHC were current or former tobacco smokers, and more than one-third met criteria for lung cancer screening. Moreover, if middle-aged adult participants (ages 40–54) continue to smoke, more than three-fourths will become eligible for screening as they age. Therefore, this high-risk, aging population may benefit from targeted interventions to build awareness of lung cancer screening and tobacco cessation. Specifically, given the premature cancer mortality faced by this population, the ideal age to begin screening patients with schizophrenia should be reassessed and merits further research to inform population-based guidelines.
As in the National Lung Screening Trial (25), former smokers in this study were significantly more likely to believe they were not in danger of developing lung cancer and reported having a lower risk of lung cancer than current smokers. Many current and former smokers underestimated their lung cancer risk; approximately three-fifths of current and former smokers believed they had a low risk of developing lung cancer, and more than half reported rarely or never worrying about developing lung cancer. Given that older smokers reported worrying less about developing lung cancer than younger smokers, those delivering interventions should consider differing cessation motivations by age.
Although both current and former smokers could benefit from increased education about their cancer risk, our findings suggest that former smokers need targeted education regarding their elevated risk of developing cancer. However, risk is a complex, abstract construct for clinicians to convey, particularly when engaging patients with schizophrenia who experience cognitive deficits in abstract thinking and working memory. Specifically, although the National Lung Screening Trial found that patients use cognitive dissonance to justify their decreased risk perception, patients with schizophrenia are at greater risk from this lowered risk perception because of cognitive impairments. Additional research is needed to determine how to best combat these deficits and facilitate increased understanding of cancer risk by patients with schizophrenia. Potential strategies include incorporating concrete examples; discussing immediate risks and benefits; and using directive language, repetition, and breaking down information into manageable pieces. Thus, to promote understanding of cancer risk among patients with schizophrenia, guidelines should account for differences in risk perceptions by smoking status. In addition, guidelines for clinicians should address building trust and promoting shared decision making for lung cancer screening to better inform individualized, effective care.
Although gaps in knowledge about lung cancer risk among patients with schizophrenia may reflect cognitive deficits in abstract thinking and executive functioning, health care clinicians may also not be communicating adequately about tobacco cessation. The National Lung Screening Trial found that 76% of participants reported that their PCPs had advised them to quit smoking (26). However, in this study, only 54% of participants reported that their PCPs advised them to quit smoking, and only 45% of participants reported that their psychiatrists advised them to quit smoking. Moreover, although participants regularly attended PCP and psychiatry appointments, few patients reported that their PCP or psychiatrist assisted with their smoking cessation.
Despite the increasing need for patients with schizophrenia to be included and actively engaged in conversations with their providers about smoking cessation and cancer screening, our findings suggest that further work is required to examine physician-level barriers and educate providers on the efficacy and benefits of smoking cessation interventions for patients with serious mental illness. In addition, the lack of clarity regarding whose role it is to provide tobacco cessation (primary care or mental health clinicians? Which team member?) poses another barrier. Practice guidelines and integrated approaches are likely needed to increase screening.
Patients from a single CMHC received primary care in 35 distinct practices, suggesting that mental health clinics and, especially, clozapine clinics, given the frequency of visits and longitudinal relationships, may have unique potential to become centralized access points for the delivery of tobacco cessation counseling and education and referrals for cancer screening to individuals with schizophrenia. Given CMS-authorized counseling about lung screening and shared decision making for eligible beneficiaries (11) and tobacco cessation treatment can be successfully incorporated into mental health settings for patients with schizophrenia (27, 28), CMHCs may have the greatest opportunity to offer these resources to a wide range of patients with little financial burden. Therefore, embedding tobacco cessation and cancer screening interventions in CMHC settings may increase access to care for an underserved population that currently experiences premature mortality from smoking-related diseases.
This is a novel study in a disadvantaged, high-risk population. To our knowledge, this is the first study to explore in-depth the barriers that may prevent patients with schizophrenia from lung cancer screening uptake. We used multiple data sources, including self-report questionnaires and medical records from psychiatry and primary care visits.
Regarding limitations, study participants were patients with schizophrenia who were prescribed clozapine in a CMHC. Psychiatrists were affiliated with an academic hospital. Moreover, patients had a high rate of access to primary care, were insured (primarily by public payers), were predominantly male and white, and had relatively high educational attainment. Thus our findings cannot be generalized to patients who access mental health care less frequently or in other care settings who may have various smoking behaviors and cancer risk perceptions. Also, although psychiatrists and psychologists assessed the survey items that were previously used in a national sample and the questions were piloted with patients with schizophrenia, the questionnaire was not validated for adults with schizophrenia.
Conclusions
To inform lung cancer screening interventions for adults with schizophrenia, research needs to elucidate barriers and facilitators of tobacco cessation and screening at the patient, clinician, and health care system levels (29). Specifically, at the patient level, targeted tobacco cessation and screening education is needed to account for the underestimation of cancer risk, cognitive deficits, negative symptoms, and social isolation common among individuals with schizophrenia. At the clinician level, mental health and primary care clinicians can benefit from training in tobacco cessation and lung cancer screening, safety of tobacco cessation medications, and clarity of role definition. At the systems level, integrated approaches to engaging patients with schizophrenia at CMHCs and during PCP visits (where and when they access clinical care) may help to mitigate disparities in cancer screening and outcomes. Future research should assess whether CMHC-based interventions can promote tobacco cessation, increase awareness of screening, and contribute to improved lung cancer survival for individuals with schizophrenia.
1 : Morbidity and Mortality in People With Serious Mental Illness. Alexandria, VA, National Association of State Mental Health Program Directors, 2006Google Scholar
2 : Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry 2013; 170:324–333Link, Google Scholar
3 : Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015; 72:1172–1181Crossref, Medline, Google Scholar
4 : Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry 2010; 196:116–121Crossref, Medline, Google Scholar
5 : Mortality in schizophrenia and schizoaffective disorder: an Olmsted County, Minnesota cohort: 1950–2005. Schizophr Res 2008; 98:287–294Crossref, Medline, Google Scholar
6 : Causes of the excess mortality of schizophrenia. Br J Psychiatry 2000; 177:212–217Crossref, Medline, Google Scholar
7 : Cancer mortality in patients with schizophrenia: an 11-year prospective cohort study. Cancer 2009; 115:3555–3562Crossref, Medline, Google Scholar
8 : Inequalities in lung cancer care of elderly patients with schizophrenia: an observational cohort study. Psychosom Med 2014; 76:215–220Crossref, Medline, Google Scholar
9 : Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409Crossref, Medline, Google Scholar
10 : Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338Medline, Google Scholar
11 Decision Memo for Screening for Lung Cancer With Low Dose Computed Tomography (LDCT). Baltimore, Centers for Medicare and Medicaid Services, 2010. www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed Dec 11, 2018Google Scholar
12 : Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol 2017; 3:1278–1281Crossref, Medline, Google Scholar
13 : Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv 2013; 64:44–50Link, Google Scholar
14 : Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry 2005; 66:183–194, quiz 147, 273–274Crossref, Medline, Google Scholar
15 : Treating Tobacco Use and Dependence: 2008 Update—Clinical Practice Guideline. Rockville, MD, US Department of Health and Human Services, 2008Google Scholar
16 : Tobacco dependence, treatment and smoke-free policies: a survey of mental health professionals’ knowledge and attitudes. Gen Hosp Psychiatry 2009; 31:576–582Crossref, Medline, Google Scholar
17 : Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database of Systematic Reviews 2013; 2:
18 : Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016; 387:2507–2520Crossref, Medline, Google Scholar
19 : Delineating responsibility: primary care provider perspective. Psychiatr Serv 2015; 66:333Link, Google Scholar
20 : Does a diagnosis of schizophrenia reduce rates of mammography screening? A Manitoba population-based study. Schizophr Res 2009; 113:95–100Crossref, Medline, Google Scholar
21 : Medical comorbidity and receipt of medical care by older homeless people with schizophrenia or depression. Psychiatr Serv 2002; 53:1456–1460Link, Google Scholar
22 : National survey of US health professionals’ smoking prevalence, cessation practices, and beliefs. Nicotine Tob Res 2010; 12:724–733Crossref, Medline, Google Scholar
23 : Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend 1994; 34:211–216Crossref, Medline, Google Scholar
24 . Base SAS 9.4 Procedures Guide. Fifth edition. Cary, NC, SAS Institute, 2017Google Scholar
25 : Risk perceptions among participants undergoing lung cancer screening: baseline results from the National Lung Screening Trial. Ann Behav Med 2009; 37:268–279Crossref, Medline, Google Scholar
26 : Primary care provider-delivered smoking cessation interventions and smoking cessation among participants in the National Lung Screening Trial. JAMA Intern Med 2015; 175:1509–1516Crossref, Medline, Google Scholar
27 : A comprehensive model for mental health tobacco recovery in New Jersey. Adm Policy Ment Health Ment Health Serv Res 2011; 38:368–383Crossref, Medline, Google Scholar
28 : Comparison of two intensities of tobacco dependence counseling in schizophrenia and schizoaffective disorder. J Subst Abuse Treat 2010; 38:384–393Crossref, Medline, Google Scholar
29 : Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry 2017; 16:30–40Crossref, Medline, Google Scholar



