Benzodiazepine Use and Misuse Among Adults in the United States
Abstract
Objective:
Goals were to determine the prevalence of benzodiazepine use (as prescribed and misuse), characterize misuse, and examine variation by age.
Methods:
A cross-sectional analysis was conducted of 2015 and 2016 National Survey on Drug Use and Health data limited to adults ≥18 (N=86,186) and data from those respondents reporting benzodiazepine use (N=10,290). Measurements included past-year prescription benzodiazepine use and misuse (“any way a doctor did not direct”), substance use disorders, mental illness, and demographic characteristics. Misuse was compared between younger (18–49) and older (≥50) adults.
Results:
A total of 30.6 million adults (12.6%) reported past-year benzodiazepine use—25.3 million (10.4%) as prescribed and 5.3 million (2.2%) misuse. Misuse accounted for 17.2% of overall use. Adults ages 50–64 had the highest prescribed use (12.9%). Those ages 18–25 had the highest misuse (5.2%), and those ages ≥65 had the lowest (.6%). Misuse and abuse of or dependence on prescription opioids or stimulants were strongly associated with benzodiazepine misuse. Benzodiazepine misuse without a prescription was the most common type of misuse, and a friend or relative was the most common source. Adults ages ≥50 were more likely than younger adults to use a benzodiazepine more often than prescribed and to use a benzodiazepine to help with sleep.
Conclusions:
Benzodiazepine use among U.S. adults was higher than previously reported, and misuse accounted for nearly 20% of use overall. Use by adults ages 50–64 now exceeds use by those ages ≥65. Patients also prescribed stimulants or opioids should be monitored for benzodiazepine misuse. Improved access to behavioral interventions for sleep or anxiety may reduce some misuse.
HIGHLIGHTS
Benzodiazepine use among 50- to 64-year-old adults now matches use among older adults (≥65).
Among those 18 to 25, benzodiazepine misuse is as prevalent as use as prescribed.
Benzodiazepines are prescribed to over 5% of the U.S. adult population, and use is growing (1–3), concentrated among middle-aged adults, for whom use increased nearly 50% from 1996 to 2013 (1). However, the prevalence of benzodiazepine use among adults ages 65 and older has been shown to be highest, at 8.6% (1). Prescribing to older adults has been considered potentially inappropriate for decades, given associated harms, including falls and fractures (4–7); however, the growth in benzodiazepine prescribing has been accompanied by increases in related adverse events for adults of all ages. One area of concern has been their combined use with opioids, given the increased risk of overdose and overdose death among opioid users coprescribed benzodiazepines (8–10). But benzodiazepines pose risks of their own. In an analysis of emergency department visits from 1999 to 2006 for poisoning from opioids, sedatives (sleep promoting), or tranquilizers (anxiolytics or muscle relaxants), the largest absolute growth was in benzodiazepine-related poisonings (11), and benzodiazepine-related overdose mortality grew nearly fivefold from 1996 to 2013 (1). Concerns related to benzodiazepine prescribing have spread beyond older adults and beyond coprescription with opioids (12).
The growth in adverse outcomes suggests that benzodiazepine prescribing and misuse in the United States have increased in tandem; however, less is known about benzodiazepine misuse. After marijuana use, prescription drug misuse—defined here as use in a manner other than prescribed or by a person to whom it was not prescribed—is the most common type of illicit drug use (13). Most recent benzodiazepine-related work has focused either on misuse in the context of opioid use (14–16) or on tranquilizer or sedative medication misuse—but not on benzodiazepines specifically (17, 18). The lack of information about misuse among older adults is particularly striking because they are prescribed benzodiazepines at the highest rates, are most at risk of related adverse events, and have higher rates of use of alcohol and other substances than prior aging cohorts (19, 20). A recent systematic review of benzodiazepine and opioid misuse among older adults (21) found just one study that estimated potential benzodiazepine misuse among older adults in the United States (22). Given their widespread use, abuse potential (23), and related risks, surprisingly little is known about benzodiazepine misuse.
Addressing the growing problem of benzodiazepine use and misuse first requires information about the current scope and nature of misuse. As a result of a 2015 redesign, the National Survey on Drug Use and Health (NSDUH) now provides detailed information regarding prescription drug use and misuse in the United States, including the type of and reasons for misuse and the source of the misused medication. We used data from the redesigned NSDUH to develop national estimates of benzodiazepine use and misuse among U.S. adults and to determine whether characteristics associated with misuse varied by age, given the unique risks of these medications to older adults and the need for targeted interventions to reduce misuse.
Methods
This analysis used data from the NSDUH, which measures the prevalence and correlates of drug use among the U.S. civilian, noninstitutionalized population (24). The survey uses a 50-state design with an independent, multistage area probability sample; is administered by RTI International; and is sponsored by the Substance Abuse and Mental Health Services Administration. Respondents complete two computer-assisted segments, one conducted by an interviewer and a second audio-assisted portion without interviewer help, intended to provide a private environment to increase the likelihood of honest reporting of illicit drug use.
NSDUH was redesigned in 2015 to collect more detailed information on the use and misuse of prescription medications, including benzodiazepines—in prior years, questions were limited to misuse exclusively (25). The pre-2015 NSDUH definition of misuse was limited to “nonmedical use,” but the 2015 definition was revised to include “in any way a doctor did not direct.” This analysis was limited to data from respondents ≥18 in the 2015 and 2016 survey years (N=86,186), the years available since the redesign.
Benzodiazepine Use or Misuse
Respondents could report benzodiazepine use through survey queries about tranquilizer or sedative use. NSDUH classifies tranquilizers as medications specifically for relief of anxiety or muscle spasms and sedatives as medications for insomnia. This analysis was limited to the 10,290 respondents who specifically reported benzodiazepine use in response to the tranquilizer and sedative items. [Additional details are provided in an online supplement to this article.]
For each medication class, NSDUH collected information on past-year use (that is, taken as prescribed) and misuse. Respondents were asked about the specific manner of misuse: without a prescription, in greater amounts or more often than prescribed, longer than prescribed, or any other use other than as prescribed. Next, they were asked about reasons for misuse: “to relax,” “to experiment,” “to get high,” “for sleep,” “for emotions,” “to counter the effect of another drug,” because they were “hooked,” or another reason. Finally, respondents were asked about the source of medication for misuse (for example, their clinician or a friend or relative). For this analysis, the misuse category identifies a respondent who reported any past-year misuse, even though that respondent may have also used the benzodiazepine as prescribed. Presence of abuse or dependence was determined by DSM-IV criteria.
Other Respondent Characteristics
We included respondent self-rating of health and past-year presence of a major depressive episode, suicidal thinking, and mental illness by using a variable developed by NSDUH based on responses to items about psychological distress, functional impairment, symptoms of a major depressive episode, and suicidal ideation (26).
We included past-year alcohol, marijuana, and heroin use or abuse or dependence; past-year use of tobacco products; and past-year use, misuse, or abuse of or dependence on prescription opioids and stimulants.
Finally, respondents provided sociodemographic information, including age, gender, race-ethnicity, and household income.
Analysis
Analyses incorporated weights, clustering, and stratification, using NSDUH design elements to account for the complex survey design and generate nationally representative estimates. After determining population characteristics among adult respondents, we estimated the prevalence of benzodiazepine use—as prescribed, misuse, and any use—among adults overall and by age group. We compared demographic and clinical characteristics of adults with and without any benzodiazepine use by using chi-square tests. We determined the magnitude of the association between respondent characteristics and benzodiazepine use by using multivariable logistic regression (0, no benzodiazepine use; 1, any benzodiazepine use), adjusting for other sociodemographic and clinical characteristics.
We then limited the analysis to respondents who reported any past-year benzodiazepine use, split into those who reported use as prescribed or misuse. We compared characteristics of each group by using chi-square tests. Next, we completed bivariate logistic regression for each characteristic (0, use as prescribed; 1, misuse), including an interaction by age (younger [18–49] versus older [≥50]), to determine whether age moderated the association of each characteristic with misuse. We used ≥50 years as the age cutoff because prescription benzodiazepine use among those in later middle age approaches that of adults ages ≥65 (1), and the youngest respondents in the Baby Boom cohort (those born in 1964) would have turned 50 just before the 2015 NSDUH. We used multivariable logistic regression to determine the characteristics associated with misuse among benzodiazepine users, adjusting for other demographic and clinical characteristics.
The final stage of analysis examined the characteristics of past-year benzodiazepine misuse overall and by age. We determined the type of, reason for, and source of misused benzodiazepine, along with the specific benzodiazepine misused.
Analyses were conducted with Stata, version 13.1, with two-sided tests and α=.05. The University of Michigan Medical School Institutional Review Board (IRB) considers analyses of deidentified, publicly available data exempt from IRB approval.
Results
Prevalence and Predictors of Any Benzodiazepine Use
An estimated 30.6 million adults per year in the United States (95% confidence interval [CI]=29.7–31.5 million) used benzodiazepines in the past year, an overall prevalence of 12.6% (CI=12.2%−12.9%), including misuse among 2.2% (CI=2.0%−2.3%) and use as prescribed among 10.4% (CI=10.1%−10.7%). Use among adults ages 50–64 was highest (Figure 1 and Table 1).
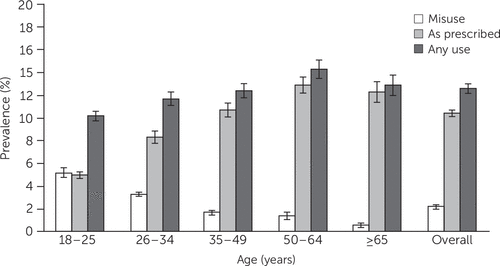
FIGURE 1. Prevalence of prescription benzodiazepine use and misuse among 2015 and 2016 NSDUH respondents (N=86,186), by age groupa
aNSDUH, National Survey on Drug Use and Health.
| Benzodiazepine useb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | All respondents (%) (N=86,186) | None (%) (N=75,896) | Any (%) (N=10,290) | Fc | df | p | AORd | 95% CI |
| Overall | 100.0 | 87.5 | 12.6 | |||||
| Demographic | ||||||||
| Age | 18.2 | 3, 138 | <.001 | |||||
| 18–25 (reference) | 14.3 | 89.8 | 10.2 | |||||
| 26–34 | 15.8 | 88.3 | 11.7 | 1.27*** | 1.18–1.36 | |||
| 35–49 | 24.9 | 87.6 | 12.4 | 1.65*** | 1.52–1.78 | |||
| 50–64 | 25.6 | 85.7 | 14.3 | 2.04*** | 1.86–2.22 | |||
| ≥65 | 19.4 | 87.1 | 12.9 | 2.28*** | 2.04–2.54 | |||
| Gender | 366.6 | 1, 50 | <.001 | |||||
| Male (reference) | 48.2 | 90.5 | 9.5 | |||||
| Female | 51.8 | 84.6 | 15.4 | 1.84*** | 1.72–1.96 | |||
| Race-ethnicity | 221.3 | 3, 124 | <.001 | |||||
| Non-Hispanic white (reference) | 64.6 | 84.6 | 15.4 | |||||
| Non-Hispanic black | 11.8 | 92.8 | 7.2 | .45*** | .40–.50 | |||
| Hispanic | 15.7 | 92.3 | 7.7 | .59*** | .54–.66 | |||
| Other | 8.0 | 93.5 | 6.5 | .45*** | .38–.53 | |||
| Education | 23.4 | 2, 84 | <.001 | |||||
| Less than high school (reference) | 13.5 | 90.1 | 9.9 | |||||
| High school graduate, some college | 56.0 | 86.6 | 13.4 | 1.28*** | 1.12–1.46 | |||
| College graduate or higher | 30.4 | 87.8 | 12.2 | 1.41*** | 1.18–1.69 | |||
| Household income | 5.2 | 3, 143 | .002 | |||||
| <$20,000 (reference) | 17.4 | 86.2 | 13.8 | |||||
| $20,000–$49,999 | 30.0 | 87.3 | 12.7 | 1.05 | .96–1.15 | |||
| $50,000–$74,999 | 16.3 | 87.9 | 12.1 | 1.05 | .94–1.17 | |||
| ≥$75,000 | 36.3 | 88.0 | 12.0 | 1.12 | .99–1.27 | |||
| Medicaid | 63.6 | 1, 50 | <.001 | |||||
| No (reference) | 85.8 | 87.9 | 12.1 | |||||
| Yes | 14.2 | 84.9 | 15.1 | 1.01 | .93–1.11 | |||
| Metropolitan area | 12.0 | 2, 94 | <.001 | |||||
| Large (reference) | 55.2 | 88.2 | 11.8 | |||||
| Small | 30.0 | 86.5 | 13.5 | 1.02 | .96–1.09 | |||
| Nonmetropolitan area | 14.8 | 86.9 | 13.1 | .96 | .87–1.05 | |||
| Past year general health | ||||||||
| Presence of a major depressive episodee | 912.5 | 1, 50 | <.001 | |||||
| No | 93.3 | 89.1 | 10.9 | |||||
| Yes | 6.7 | 66.4 | 33.6 | |||||
| Suicidal ideatione | 857.7 | 1, 50 | <.001 | |||||
| No | 96.0 | 88.3 | 11.7 | |||||
| Yes | 4.0 | 68.6 | 31.4 | |||||
| Presence of mental illness | 215.9 | 2, 98 | <.001 | |||||
| None (reference) | 81.9 | 90.9 | 9.1 | |||||
| Mild | 9.2 | 78.5 | 21.5 | 1.93*** | 1.74–2.15 | |||
| Moderate | 4.8 | 70.7 | 29.3 | 2.47*** | 2.20–2.76 | |||
| Severe | 4.2 | 58.5 | 41.5 | 3.80*** | 3.40–4.26 | |||
| Health self-rating | 961.7 | 3, 142 | <.001 | |||||
| Excellent or very good (reference) | 56.8 | 90.2 | 9.8 | |||||
| Good | 29.2 | 86.4 | 13.6 | 1.26*** | 1.16–1.38 | |||
| Fair or poor | 14.0 | 78.4 | 21.6 | 1.78*** | 1.59–2.00 | |||
| Past-year substance use | ||||||||
| Tobacco | 302.1 | 1, 50 | <.001 | |||||
| None (reference) | 69.2 | 89.5 | 10.5 | |||||
| Any | 30.8 | 83.0 | 17.0 | 1.27*** | 1.16–1.38 | |||
| Alcohol | 151.9 | 2, 93 | <.001 | |||||
| None (reference) | 30.3 | 89.5 | 10.5 | |||||
| Any | 63.5 | 87.6 | 12.4 | 1.10* | 1.00–1.21 | |||
| Abuse or dependence | 6.2 | 75.9 | 24.1 | 1.53*** | 1.35–1.73 | |||
| Marijuana | 521.5 | 2, 96 | <.001 | |||||
| None (reference) | 86.1 | 89.2 | 10.8 | |||||
| Any | 12.5 | 77.3 | 22.7 | 1.81*** | 1.66–1.98 | |||
| Abuse or dependence | 1.4 | 69.7 | 30.3 | 1.97*** | 1.65–2.34 | |||
| Heroin | 292.1 | 2, 96 | <.001 | |||||
| None (reference) | 99.6 | 87.6 | 12.4 | |||||
| Any | .1 | 44.3 | 55.7 | 2.92** | 1.49–5.75 | |||
| Abuse or dependence | .2 | 35.8 | 64.2 | 2.65*** | 1.88–3.74 | |||
| Prescription opioid | 1094.0 | 3, 137 | <.001 | |||||
| None (reference) | 63.2 | 93.2 | 6.8 | |||||
| Prescription use | 32.3 | 80.0 | 20.0 | 2.54*** | 2.39–2.71 | |||
| Misuse | 3.8 | 64.5 | 35.5 | 4.21*** | 3.66–4.85 | |||
| Abuse or dependence | .7 | 40.1 | 59.9 | 7.34*** | 5.51–9.76 | |||
| Prescription stimulant | 565.5 | 3, 133 | <.001 | |||||
| None (reference) | 93.3 | 88.9 | 11.1 | |||||
| Prescription use | 4.6 | 70.2 | 29.8 | 2.11*** | 1.89–2.36 | |||
| Misuse | 1.9 | 62.6 | 37.4 | 2.71*** | 2.31–3.18 | |||
| Abuse or dependence | .2 | 45.7 | 54.3 | 2.21*** | 1.48–3.29 | |||
TABLE 1. Overall characteristics and predictors of any past-year benzodiazepine use among all adult 2015 and 2016 NSDUH respondentsa
Women and non-Hispanic white respondents reported the highest rates of any past-year use. In the adjusted logistic regression model (Table 1), female gender, older age, and more education were all associated with increased odds of use, and respondents’ race-ethnicity other than non-Hispanic white was associated with lower odds of any benzodiazepine use.
The presence of past-year mental illness was associated with increased odds of any use, as was worse self-rated health. In almost every instance, past-year use, misuse, or abuse of or dependence on tobacco, alcohol, marijuana, heroin, prescription opioids, or prescription stimulants was associated with any benzodiazepine use. Among all substances, prescription opioids were most strongly associated with benzodiazepine use at every level, from use as prescribed through abuse or dependence.
Prevalence and Predictors of Benzodiazepine Misuse
Among respondents who reported any benzodiazepine use, 25.3 million (CI=24.5–26.1 million) reported use as prescribed by their clinician, and 5.3 million (CI=5.0–5.6 million) reported misuse. Use as prescribed was highest among adults ages 50–64 (Figure 1 and Table 2). Misuse was highest among the youngest adults and decreased with age. Most benzodiazepine use among respondents ages 18–25 was misuse (5.2%, CI=4.8%−5.6%). In contrast, misuse was reported by just .6% (CI=.4%–.8%) of adults ages ≥65.
| All respondents (%) (N=86,186)b | Benzodiazepine users (%) (N=10,290)c | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | As prescribed (N=7,757) | Misuse (N=2,533) | As prescribed (N=7,757) | Misuse (N=2,533) | Fd | df | p | AORe | 95% CI |
| Overall | 10.4 | 2.2 | 82.8 | 17.2 | |||||
| Demographic | |||||||||
| Age | 188.2 | 3, 139 | <.001 | ||||||
| 18–25 (reference) | 5.0 | 5.2 | 49.0 | 51.0 | |||||
| 26–34 | 8.3 | 3.3 | 71.4 | 28.6 | .55** | .46–.66 | |||
| 35–49 | 10.7 | 1.7 | 86.2 | 13.8 | .33** | .27–.41 | |||
| 50–64 | 12.9 | 1.4 | 90.3 | 9.7 | .33** | .26–.43 | |||
| ≥65 | 12.3 | .6 | 95.6 | 4.4 | .23** | .15–.34 | |||
| Gender | 102.3 | 1, 50 | <.001 | ||||||
| Male (reference) | 7.3 | 2.3 | 76.3 | 23.7 | |||||
| Female | 13.3 | 2.1 | 86.5 | 13.5 | .74** | .61–.90 | |||
| Race-ethnicity | 4.9 | 3, 146 | .003 | ||||||
| Non-Hispanic white (reference) | 12.9 | 2.5 | 83.6 | 16.4 | |||||
| Non-Hispanic black | 5.7 | 1.5 | 79.6 | 20.4 | 1.25 | .94–1.65 | |||
| Hispanic | 6.1 | 1.6 | 78.8 | 21.2 | 1.05 | .79–1.38 | |||
| Other | 5.4 | 1.1 | 82.4 | 17.6 | .95 | .67–1.35 | |||
| Education | 9.4 | 2, 100 | <.001 | ||||||
| Less than high school (reference) | 8.1 | 1.9 | 81.3 | 18.7 | |||||
| High school graduate, some college | 10.9 | 2.5 | 81.3 | 18.7 | .99 | .77–1.26 | |||
| College graduate or higher | 10.5 | 1.7 | 86.2 | 13.8 | .90 | .67–1.22 | |||
| Household income | 9.4 | 3, 140 | <.001 | ||||||
| <$20,000 (reference) | 10.9 | 3.0 | 78.6 | 21.4 | |||||
| $20,000–$49,999 | 10.4 | 2.3 | 81.9 | 18.1 | .98 | .79–1.23 | |||
| $50,000–$74,999 | 10.3 | 1.8 | 85.0 | 15.0 | .77 | .58–1.03 | |||
| ≥$75,000 | 10.2 | 1.8 | 84.9 | 15.1 | .84 | .67–1.06 | |||
| Medicaid | 7.0 | 1, 50 | .01 | ||||||
| No (reference) | 10.1 | 2.0 | 83.3 | 16.7 | |||||
| Yes | 12.1 | 3.0 | 80.2 | 19.8 | .99 | .79–1.23 | |||
| Metropolitan area | 4.8 | 2, 95 | .01 | ||||||
| Large (reference) | 9.7 | 2.1 | 82.0 | 18.0 | |||||
| Small | 11.1 | 2.4 | 82.5 | 17.5 | 1.02 | .83–1.25 | |||
| Nonmetropolitan | 11.3 | 1.8 | 86.0 | 14.0 | .84 | .67–1.06 | |||
| Past year clinical | |||||||||
| Presence of major depressive episodef | 20.9 | 1, 50 | <.001 | ||||||
| No | 9.2 | 1.8 | 83.8 | 16.2 | |||||
| Yes | 26.4 | 7.2 | 78.5 | 21.5 | |||||
| Suicidal ideationf | 92.9 | 1, 50 | <.001 | ||||||
| No | 9.9 | 1.8 | 84.3 | 15.7 | |||||
| Yes | 21.7 | 9.7 | 69.1 | 30.9 | |||||
| Presence of mental illness | 15.1 | 3, 139 | <.001 | ||||||
| None (reference) | 7.7 | 1.4 | 84.8 | 15.2 | |||||
| Mild | 18.0 | 3.6 | 83.5 | 16.5 | .90 | .72–1.12 | |||
| Moderate | 22.8 | 6.5 | 77.9 | 22.1 | 1.24 | .93–1.63 | |||
| Severe | 31.7 | 9.8 | 77.3 | 22.7 | 1.19 | .93–1.53 | |||
| Health self-rating | 30.1 | 2, 96 | <.001 | ||||||
| Excellent or very good (reference) | 7.8 | 2.0 | 79.4 | 20.6 | |||||
| Good | 11.2 | 2.4 | 82.7 | 17.3 | .85 | .70–1.04 | |||
| Fair or poor | 19.2 | 2.3 | 89.2 | 10.8 | .61** | .47–.80 | |||
| Other past-year substance use | |||||||||
| Tobacco | 565.4 | 1, 50 | <.001 | ||||||
| None (reference) | 9.5 | 1.0 | 90.7 | 9.3 | |||||
| Any | 12.3 | 4.8 | 71.9 | 28.1 | 1.41** | 1.17–1.70 | |||
| Alcohol | 199.5 | 2, 90 | <.001 | ||||||
| None (reference) | 9.8 | .8 | 92.8 | 7.2 | |||||
| Any | 10.4 | 2.0 | 83.5 | 16.5 | 1.42* | 1.08–1.87 | |||
| Abuse or dependence | 13.8 | 10.3 | 57.2 | 42.8 | 2.31** | 1.73–3.09 | |||
| Marijuana | 558.3 | 2, 99 | <.001 | ||||||
| None (reference) | 9.8 | 1.0 | 90.7 | 9.3 | |||||
| Any | 14.4 | 8.3 | 63.3 | 36.7 | 1.88** | 1.58–2.24 | |||
| Abuse or dependence | 11.7 | 18.6 | 38.7 | 61.3 | 2.39** | 1.62–3.52 | |||
| Heroin | 90.3 | 2, 86 | <.001 | ||||||
| None (reference) | 10.3 | 2.0 | 83.6 | 16.4 | |||||
| Any | 26.6 | 29.2 | 47.7 | 52.3 | .91 | .41–2.04 | |||
| Abuse or dependence | 23.5 | 40.8 | 36.5 | 63.5 | 1.20 | .76–1.91 | |||
| Prescription opioid | 462.0 | 3, 144 | <.001 | ||||||
| None (reference) | 5.9 | .9 | 86.2 | 13.8 | |||||
| Prescription use | 18.3 | 1.7 | 91.5 | 8.5 | .69** | .57–.84 | |||
| Misuse | 15.6 | 19.9 | 44.1 | 55.9 | 4.11** | 3.26–5.18 | |||
| Abuse or dependence | 24.4 | 35.6 | 40.7 | 59.3 | 4.81** | 3.45–6.72 | |||
| Prescription stimulant | 410.6 | 3, 127 | <.001 | ||||||
| None (reference) | 9.6 | 1.5 | 86.8 | 13.2 | |||||
| Prescription use | 24.1 | 5.7 | 80.9 | 19.1 | .90 | .74–1.09 | |||
| Misuse | 13.3 | 24.1 | 35.6 | 64.4 | 2.46** | 1.85–3.27 | |||
| Abuse or dependence | 14.7 | 39.6 | 27.1 | 72.9 | 3.40** | 1.72–6.70 | |||
TABLE 2. Past-year prevalence of use and misuse of prescription benzodiazepines among adult NSDUH respondents and predictors of benzodiazepine misuse, by characteristica
Bivariate logistic regressions testing for a moderating effect of age on the associations between respondent characteristics and misuse found a statistically significant interaction in just three instances (Table 2). Even though age itself was strongly associated with lower odds of misuse, because of the minimal evidence for a moderating effect, characteristic × age interactions were not included in the multivariable model. Females had lower odds than males of misuse; however, apart from age, no other demographic characteristic was associated with misuse. Fair or poor health self-rating was associated with lower odds of misuse, whereas any level of marijuana or alcohol use was associated with increased odds of benzodiazepine misuse. Prescription opioid use as prescribed was associated with lower odds of benzodiazepine misuse, whereas opioid misuse and abuse or dependence were the characteristics most strongly associated with benzodiazepine misuse.
Characteristics of Misuse Among Younger and Older Adults
The most common type of benzodiazepine misuse overall was use without a prescription, although this type of misuse use was less likely to be reported by respondents ages ≥50, compared with younger adults (ages 18–49) (Table 3). Compared with younger adults, older respondents were more likely to report using their benzodiazepine more often than prescribed.
| Age group (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Overall | 18–49 (N=2,357) | ≥50 (N=176) | ORb | 95% CI | p |
| Type of misuse | ||||||
| Without a prescription | 73.9 | 78.4 | 56.4 | .36 | .25 to .51 | <.001 |
| Used in greater amounts than prescribed | 16.0 | 15.0 | 20.2 | 1.44 | .86 to 2.40 | .16 |
| Used more often than prescribed | 10.1 | 8.1 | 17.9 | 2.47 | 1.57 to 3.90 | <.001 |
| Used longer than prescribed | 4.9 | 4.7 | 5.7 | 1.24 | .62 to 2.48 | .53 |
| Used in other ways other than as directed | 18.7 | 17.6 | 23.2 | 1.41 | .90 to 2.21 | .13 |
| Main reason for misuse | ||||||
| Relax, relieve tension | 47.1 | 48.6 | 41.7 | .76 | .53 to 1.08 | .12 |
| Experiment | 6.0 | 6.5 | 4.0 | .59 | .21 to 1.68 | .28 |
| Get high | 11.6 | 13.9 | 3.0 | .19 | .07 to 56 | .003 |
| Help with sleep | 26.6 | 22.4 | 41.7 | 2.48 | 1.83 to 3.35 | <.001 |
| Help with feelings, emotions | 10.1 | 10.4 | 9.1 | .86 | .43 to 1.76 | .68 |
| Increase or decrease effects of another drug | 1.6 | 1.7 | .9 | .51 | .11 to 2.35 | .38 |
| Hooked or have to have it | .6 | .5 | .9 | 1.89 | .17 to 20.72 | .60 |
| Source of medication for misuse | ||||||
| 1 clinician | 19.5 | 14.3 | 38.4 | 3.73 | 2.65 to 5.26 | <.001 |
| ≥2 clinicians | .9 | .6 | 1.9 | 3.27 | .87 to 12.29 | .08 |
| Stolen from health care setting | .1 | .2 | .0 | |||
| Free from friend or relative | 53.2 | 55.4 | 45.1 | .66 | .44 to .99 | .04 |
| Bought from friend or relative | 12.3 | 14.2 | 5.6 | .35 | .15 to .84 | .02 |
| Stolen from friend or relative | 3.3 | 3.2 | 3.5 | 1.08 | .31 to 3.81 | .90 |
| Bought from dealer or stranger | 7.7 | 8.9 | 3.1 | .33 | .10 to 1.07 | .06 |
| Medication misused | ||||||
| Alprazolam | 75.1 | 80.3 | 56.4 | .32 | .21 to.49 | <.001 |
| Lorazepam | 15.4 | 12.6 | 26.0 | 2.44 | 1.54 to 3.87 | <.001 |
| Clonazepam | 20.0 | 21.7 | 13.6 | .57 | .33 to 1.00 | .05 |
| Diazepam | 20.5 | 17.8 | 30.5 | 2.04 | 1.21 to 3.43 | .009 |
| Other characteristics of benzodiazepine misuse | ||||||
| Mean days of misuse in past month | 5.4 | 5.0 | 7.1 | 2.10 | –.30 to 4.51 | .09 |
| Meets past-year criteria for abuse (reference: no) | 4.6 | 5.0 | 3.1 | .60 | .20 to 1.78 | .35 |
| Meets past-year criteria for dependence (reference: no) | 6.8 | 6.8 | 6.6 | .97 | .50 to 1.90 | .94 |
TABLE 3. Characteristics of benzodiazepine misuse among 2015 and 2016 NSDUH respondents (N=2,533), by age groupa
The most common reason for misuse overall was to relax or relieve tension, followed by to help with sleep. Older adults were significantly more likely than younger adults to endorse misuse to help with sleep, and they were much less likely to report misuse to get high.
The most common source of the misused medication for both younger and older groups was from a friend or relative. When all sources of benzodiazepines were combined—free, bought, or stolen—a friend or relative was the source for nearly 70% of respondents reporting misuse. The next most common source was a single clinician.
Alprazolam was the most common benzodiazepine misused. Compared with younger adults, older adults were more likely to misuse lorazepam or diazepam. Respondents who reported misuse did so on 5.4 days (SE=.3) in the past month. Among those with benzodiazepine misuse, 4.6% (CI=3.7%−5.6%) and 6.8% (CI=5.6%−8.2%) met criteria for past-year abuse and past-year dependence, respectively.
Discussion
In this cross-sectional analysis of survey data from U.S. adults, the annual prevalence of benzodiazepine use, when both prescription use and misuse were included, was 12.6% and exceeded 15% among women and non-Hispanic white respondents. By age, the highest rate of overall benzodiazepine use was among adults ages 50–64. More than 2% of respondents overall endorsed misuse, which was highest among the youngest adults (ages 18–25), for whom misuse exceeded as-prescribed use. In contrast, adults ages ≥65 had the lowest prevalence of misuse (.6%).
To our knowledge, this is the first analysis of benzodiazepine use in the United States to find that the highest prevalence of use is no longer among adults ages ≥65. Although use among those ages ≥65 has not been declining (1, 3), the decades of evidence regarding safety concerns (4, 7, 27) and professional guidelines (6, 28) recommending limited use among older adults may have helped slow growth. In contrast, the aging Baby Boomers—who constituted nearly the entire 50–64 age group in this analysis—have higher rates of alcohol and other substance use, compared with previous cohorts of aging adults (19, 20, 29). The high level of use among these late-middle-age adults means that potentially inappropriate prescribing of benzodiazepines to older adults may continue as this cohort ages.
The NSDUH redesign affords new insights into the nature of benzodiazepine misuse, which accounted for nearly 20% of all use among adults but which was much more common among younger adults. Although age largely did not moderate the patient characteristics associated with misuse, the nature of misuse varied between younger (ages 18–49) and older (ages ≥50) adults. Misuse without a prescription was the most common type of misuse, although this was more common among younger adults; older adults were more likely to use their benzodiazepine more often than prescribed. Misuse to help relax and to help sleep were the main reasons for misuse among both age groups, although sleep was a relatively larger driver of misuse among older adults. Relatively little misuse was for experimentation or to get high, and few respondents who reported benzodiazepine misuse met criteria for past-year abuse or dependence.
Taken together, these misuse findings raise questions about the underlying contributors to misuse as defined and identified in the NSDUH. Younger adults are more likely than other age groups to lack health insurance (30), whereas the most common reasons for misuse (for example, to relax or relieve tension) were reasons for which a clinician might prescribe a benzodiazepine or refer for behavioral treatment. Therefore, a significant proportion of NSDUH-defined “misuse” could reflect use for untreated symptoms among those with poor access to care—specifically, for behavioral treatments for insomnia (31, 32) or anxiety disorders (33, 34). The development of interventions, such as Web-based cognitive-behavioral therapy for insomnia, may help increase access to benzodiazepine alternatives for those who lack access to providers or insurance or both (35).
Clinicians should recognize their role as a source of misused benzodiazepines, either through medication that they prescribe but that is used other than as instructed or as the source of prescribed medication given for misuse to a friend or relative. In addition to being mindful of their role as a potential source of misused medications, clinicians have an important role in understanding the reason for their patients’ misuse to determine the appropriate intervention. If patients are consuming prescribed medication faster than expected, clinicians should determine the reason. Is it for inadequate symptom control? For additional indications—for example, prescribed for anxiety but also used for sleep? Is a patient allowing another family member to use some? Some misuse may be for symptoms appropriately treated by a benzodiazepine, but clinicians should be mindful of other potential reasons for misuse. An uninsured young person may use an older relative’s prescribed benzodiazepine for insomnia rather than to get high, but this certainly was not the intention of the prescribing clinician.
Other substance use disorders were strongly associated with benzodiazepine use and misuse. Benzodiazepine misuse was most strongly associated with misuse of and abuse of or dependence on prescription opioids and stimulants. Prescription drug monitoring programs are an important tool for clinicians to understand which of their patients may be misusing other medications and would thus be at high risk of benzodiazepine misuse. The association of alcohol abuse or dependence with increased odds of benzodiazepine misuse is particularly concerning in light of the increased potential for fatal poisoning when these substances are combined (36, 37). This has received much less attention than the opioid-benzodiazepine combination, even though alcohol use disorders are more prevalent than prescription opioid use disorders.
Our findings that women, older respondents, and non-Hispanic white respondents reported higher rates of benzodiazepine use are consistent with previous work (1, 2, 38–40). In contrast, women and older respondents were less likely than men and younger respondents to report misuse. This may further support the hypothesis that higher misuse is partly a function of limited access to a prescribed option—limited for younger individuals because of lack of insurance and for men because of minimal disclosure of mental health concerns to providers (41). However, higher misuse was not found among racial and ethnic minority groups, even though they have limited access to specialty mental health care and lower rates of insurance, compared with non-Hispanic whites (30).
Our estimate of the annual prevalence of any benzodiazepine use—12.6% overall and 10.4% as prescribed—is higher than other recent results. A study using data from the Medical Expenditure Panel Survey (MEPS) found that 5.6% of adults filled a benzodiazepine prescription in 2013 (1), and an analysis of data from the 2013–2014 National Health and Nutrition Examination Survey (NHANES) found benzodiazepine use among 4.2% of adults (42). Survey differences may partly account for the different estimates: NHANES assesses medication use in the past 30 days (43), whereas MEPS samples households, with information often provided by a single knowledgeable informant (44). MEPS respondents may underestimate the number of unique medications used in a year, specifically underreporting those used for a shorter duration or with fewer fills (45), which is how benzodiazepines may be prescribed. Finally, NSDUH is the only survey to include misuse. Our estimate of misuse is similar to an NSDUH estimate from 2002–2004 (17), although that analysis examined tranquilizers and sedatives overall and was not limited to benzodiazepines.
Our analysis had a number of limitations. NSDUH is cross-sectional, and because of the 2015 redesign, we could not track trends in misuse over time. There was a potential for nonresponse bias. NSDUH response rates have been declining, although this is unfortunately true for several federally administered national surveys (46). NSDUH is nationally representative but of the civilian population and thus does not include active-duty members of the military or institutionalized adults.
Conclusions
The study found a prevalence of benzodiazepine use among U.S. adults that is higher than previously reported. Misuse was highest among the youngest adults. Overall use among adults ages 50–64 was found to exceed that among adults ages ≥65. At the policy level, more widespread insurance coverage and access to behavioral treatments may reduce benzodiazepine use and misuse—some misuse may reflect limited access to health care generally and behavioral treatments specifically. Although clinicians should be mindful of the potential for benzodiazepine misuse among patients with any level of substance use, findings indicated that prescription opioid and stimulant use disorders were most strongly associated with benzodiazepine misuse.
1 : Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health 2016; 106:686–688Crossref, Medline, Google Scholar
2 : Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142Crossref, Medline, Google Scholar
3 : National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry 2017; 78:e363–e371Crossref, Medline, Google Scholar
4 : Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med 2009; 169:1952–1960Crossref, Medline, Google Scholar
5 : Inappropriate medication use in community-residing older persons. Arch Intern Med 1994; 154:2195–2200Crossref, Medline, Google Scholar
6 : American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246Crossref, Medline, Google Scholar
7 : Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA 1989; 262:3303–3307Crossref, Medline, Google Scholar
8 : Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ 2015; 350:h2698Crossref, Medline, Google Scholar
9 : Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017; 356:j760Crossref, Medline, Google Scholar
10 : Physician prescribing of opioids to patients at increased risk of overdose from benzodiazepine use in the United States. JAMA Psychiatry 2018; 75:623–630Crossref, Medline, Google Scholar
11 : Hospitalizations for poisoning by prescription opioids, sedatives, and tranquilizers. Am J Prev Med 2010; 38:517–524Crossref, Medline, Google Scholar
12 : Our other prescription drug problem. N Engl J Med 2018; 378:693–695Crossref, Medline, Google Scholar
13 : Why Do Adults Misuse Prescription Drugs? CBHSQ Report. Rockville, MD, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2017. https://www.ncbi.nlm.nih.gov/books/NBK458284Google Scholar
14 : Trends in intentional abuse or misuse of benzodiazepines and opioid analgesics and the associated mortality reported to poison centers across the United States from 2000 to 2014. Clin Toxicol 2018; 61:1–8Google Scholar
15 : Prevalence and correlates of benzodiazepine use and misuse among young adults who use prescription opioids non-medically. Drug Alcohol Depend 2018; 183:73–77Crossref, Medline, Google Scholar
16 : Assessment of risk behaviors in patients with opioid prescriptions: a study of Indiana’s INSPECT data. Am J Addict 2017; 26:822–829Crossref, Medline, Google Scholar
17 : Non-medical use, abuse and dependence on sedatives and tranquilizers among US adults: psychiatric and socio-demographic correlates. Drug Alcohol Depend 2007; 90:280–287Crossref, Medline, Google Scholar
18 : Trends in older adult nonmedical prescription drug use prevalence: results from the 2002–2003 and 2012–2013 National Survey on Drug Use and Health. Addict Behav 2016; 60:219–222Crossref, Medline, Google Scholar
19 : The epidemiology of substance use and disorders among middle-aged and elderly community adults: National Survey on Drug Use and Health. Am J Geriatr Psychiatry 2009; 17:237–245Crossref, Medline, Google Scholar
20 : Nonprescription use of pain relievers by middle-aged and elderly community-living adults: National Survey on Drug Use and Health. J Am Geriatr Soc 2009; 57:1252–1257Crossref, Medline, Google Scholar
21 : A systematic review of opioid and benzodiazepine misuse in older adults. Am J Geriatr Psychiatry 2016; 24:949–963Crossref, Medline, Google Scholar
22 : Assessing opioid shopping behaviour: a large cohort study from a medication dispensing database in the US. Drug Saf 2012; 35:325–334Crossref, Medline, Google Scholar
23 : Benzodiazepine use, abuse, and dependence. J Clin Psychiatry 2005; 66(suppl 2):28–33Crossref, Medline, Google Scholar
24 2016 National Survey on Drug Use and Health Public Use File Codebook. Rockville, MD, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2017Google Scholar
25 2015 National Survey on Drug Use and Health: Summary of the Effects of the 2015 NSDUH Questionnaire Redesign: Implications for Data Users. Rockville, MD, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2016Google Scholar
26 2015 National Survey on Drug Use and Health: Methodological Summary and Definitions. Rockville, MD, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2016Google Scholar
27 : Explicit criteria for determining potentially inappropriate medication use by the elderly: an update. Arch Intern Med 1997; 157:1531–1536Crossref, Medline, Google Scholar
28 Ten Things Physicians and Patients Should Question. Philadelphia, American Board of Internal Medicine Foundation, Choosing Wisely Initiative, 2015. http://www.choosingwisely.org/societies/american-geriatrics-society. Accessed Aug 2, 2017Google Scholar
29 : Cross-Cohort Differences in Health on the Verge of Retirement. Cambridge, MA, National Bureau of Economic Research, 2006Crossref, Google Scholar
30 HI-01: Health Insurance Coverage Status and Type of Coverage by Selected Characteristics. Washington, DC, US Census Bureau, 2017. https://www.census.gov/data/tables/time-series/demo/income-poverty/cps-hi/hi-01.html. Accessed Aug 14, 2018Google Scholar
31 : Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016; 165:125–133Crossref, Medline, Google Scholar
32 : Clinical management of insomnia disorder. JAMA 2017; 318:1973–1974Crossref, Medline, Google Scholar
33 : Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol 2014; 28:403–439Crossref, Medline, Google Scholar
34 : Treating anxiety in 2017: optimizing care to improve outcomes. JAMA 2017; 318:235–236Crossref, Medline, Google Scholar
35 : Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: a randomized clinical trial. JAMA Psychiatry 2017; 74:68–75Crossref, Medline, Google Scholar
36 : Toxicological interactions between alcohol and benzodiazepines. J Toxicol Clin Toxicol 2002; 40:69–75Crossref, Medline, Google Scholar
37 : Benzodiazepines and alcohol. J Psychiatr Res 1990; 24(suppl 2):121–127Crossref, Medline, Google Scholar
38 : No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc 2016; 64:2546–2553Crossref, Medline, Google Scholar
39 : Sedative, hypnotic, and antianxiety medication use in an aging cohort over ten years: a racial comparison. J Am Geriatr Soc 2000; 48:1073–1079Crossref, Medline, Google Scholar
40 : Examining racial/ethnic differences in patterns of benzodiazepine prescription and misuse. Drug Alcohol Depend 2018; 187:29–34Crossref, Medline, Google Scholar
41 : Help-seeking behaviour in men and women with common mental health problems: cross-sectional study. Br J Psychiatry 2005; 186:297–301Crossref, Medline, Google Scholar
42 : Long-term use of benzodiazepines and nonbenzodiazepine hypnotics, 1999–2014. Psychiatr Serv 2018; 69:235–238Link, Google Scholar
43 National Health and Nutrition Examination Survey: 2013–2014 Data Documentation, Codebook, and Frequencies (Prescription Medications [RXQ_RX_H]). Atlanta, Centers for Disease Control and Prevention, National Center for Health Statistics, 2016. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/RXQ_RX_H.htm. Accessed Aug 15, 2018Google Scholar
44 Medical Expenditure Panel Survey: Frequently Asked Questions. Rockville, MD, Agency for Healthcare Research and Quality. https://meps.ahrq.gov/communication/household_participant_faqs.jsp#7. Accessed Aug 15, 2018Google Scholar
45 : Implications of the accuracy of MEPS prescription drug data for health services research. Inquiry 2011; 48:242–259Crossref, Medline, Google Scholar
46 : Declining Response Rates in Federal Surveys: Trends and Implications. Washington, DC, Mathematica Policy Research, 2016. https://aspe.hhs.gov/system/files/pdf/255531/Decliningresponserates.pdfGoogle Scholar



