Physicians as a Source of Medications for Nonmedical Use: Comparison of Opioid Analgesic, Stimulant, and Sedative Use in a National Sample
Abstract
Objective:
An estimated 6.5 million Americans engage in nonmedical use (NMU) of prescription medications. Physicians can be important targets for interventions to reduce NMU, but little is known about which individuals engaging in NMU receive medications from a physician versus other sources.
Methods:
Cross-sectional data from the National Survey on Drug Use and Health, 2006–2013, were analyzed. The sample included 34,690 persons ages 12 and older who reported prior-year NMU. Individuals identified medications used that were not prescribed to them or that they took only for the experience and feelings that they caused and identified the most recent source of each NMU medication. The sample was stratified into groups by medication obtained for NMU (opioid analgesics, stimulants, tranquilizers or sedatives, and multiple medications) and the proportion receiving each medication from a physician. Logistic regression was used to examine sociodemographic, health status, and substance use correlates of reporting a physician source for NMU medications.
Results:
The percentage that received medications from a physician varied by group: opioid analgesics, 23.7%; multiple medications, 20.1%; tranquilizers-sedatives, 11.9%; and stimulants, 10.4%. Across groups, positive correlates of reporting a physician as a source of NMU medications included male gender, non-Hispanic black race, receipt of mental health treatment, and more frequent NMU. Individuals reporting use of illicit drugs were less likely to receive medications for NMU from a physician.
Conclusions:
Among individuals who engaged in NMU, those with the greatest medical vulnerability were more likely to have a physician source. Clinical interventions to identify harmful use can play an important role in reducing NMU.
An estimated 6.5 million American adults and adolescents engage in nonmedical use (NMU) of prescription medications, and for more than one-quarter of these individuals, their use meets criteria for abuse or dependence (1). NMU encompasses many behaviors and motivations. Some individuals engage in NMU for recreational purposes. Others have medical conditions for which the medication is indicated but also use the medication for purposes beyond those indicated by their physician (2). Opioid analgesics are the medications most commonly used for nonmedical reasons, followed by stimulants, sedatives (for example, benzodiazepines), and tranquilizers (for example, barbiturates). NMU is a well-established risk factor for development of a substance use disorder and for adverse events, such as falls, motor vehicle accidents, and overdose (3–7).
As gatekeepers of prescription medications, prescribing physicians may be important targets for interventions to reduce NMU. Physicians are generally responsible for maintaining contact with patients to ensure that prescribed medications are used appropriately, controlling medication dosage, and monitoring patients for warning signs of addiction. Determining which patients are most likely to obtain medications for NMU from physicians can identify subgroups that are most amenable to clinical interventions (that is, those with frequent physician contact). Identification of subgroups at risk of NMU can also help insurance programs better understand whether their populations are likely to obtain NMU medications from physicians so that utilization management tools can be implemented to restrict problematic prescribing (8). Finally, this information can inform nascent efforts to bridge general medical and behavioral health services (9) by suggesting which patients can benefit most from care coordination related to addictive medication use.
Existing research is limited on the role of physicians as a medication source for NMU. For opioid analgesics, research indicates that most persons engaging in NMU receive medications from friends or family members and that physicians are a more common source for those engaging in high-volume NMU (10). For stimulants, the focus has been on adolescent and college-age individuals. Friends are the most common source for stimulants (11,12), but as with opioid analgesics, individuals with more problematic and frequent NMU are more likely to report physicians as a source (13). Little attention has been paid to individuals who use tranquilizers and sedatives and to those concurrently using multiple classes of medications.
To our knowledge, no prior study has compared factors associated with obtaining specific types of medications for NMU from a physician versus other sources. Our objective was to quantify levels of specific medication types—opioid analgesics, tranquilizers or sedatives, stimulants, and multiple types of medications—obtained for NMU from a physician and identify correlates of obtaining medications for NMU from a physician. Existing research does not indicate whether correlates are common across types of medications. We hypothesized that for all medication types, individuals likely to have greater contact with a physician would have easier access to a physician source (including individuals with health insurance coverage and with poor health status). Conversely, we hypothesized that individuals using illicit drugs may be less likely to obtain prescription medications for NMU from a physician because they have established access to illicit suppliers, such as drug dealers or friends using illicit substances.
Methods
Data
We conducted an analysis of pooled data spanning 2006 to 2013 from the National Survey on Drug Use and Health (NSDUH). NSDUH annually interviews about 70,000 noninstitutionalized individuals ages 12 and older. Respondents are interviewed in their homes; to ensure confidentiality, most directly enter responses about substance use into a computer (14). The NSDUH collects demographic and socioeconomic information. Overall response rates varied from 60.2% to 67.0% during our study period (15).
For each type of prescription medication, respondents were shown cards depicting commonly prescribed medications and asked whether in the past 12 months they used any of the medications on the cards “not prescribed for you or that you took only for the experience or feeling they caused.” Among 447,196 respondents, we identified 39,732 individuals with NMU in the prior 12 months (8.9%, unweighted percentage).
Our outcome variable measured the most recent source of medication used nonmedically. We focused primarily on individuals who obtained medications from a physician rather than from another source. However, we descriptively compared rates for the two most prominent nonphysician sources—a friend or relative (that is, either bought, took without asking, or was given for free) or some other source (that is, from a dealer, stole from a hospital or doctor’s office, bought on the Internet, wrote a fake prescription, or some other way). Medication source was missing for 12.6% of the sample, which resulted in a final sample of 34,690 respondents. We divided our sample by type of medication obtained for NMU in the prior year: individuals who exclusively used opioid analgesics (N=18,219), tranquilizers or sedatives (N=3,651), and stimulants (N=2,592) and those who used more than one of these medications (N=10,228). For those who used multiple medications, we classified individuals as receiving a drug from a physician if they reported a physician as a source for any of the drugs they reported using.
We focused on explanatory variables likely to be both associated with source of medication and informative for policy interventions: demographic factors (age, sex, and race-ethnicity), socioeconomic status (post–high school education and low-income status), insurance coverage (private insurance, Medicaid only, Medicare only or other, dual Medicaid and Medicare, and uninsured), self-rated fair or poor health, substance use behavior (frequency of NMU and concurrent use of illicit drugs), criminal activity (probation or parole and sells drugs), and behavioral health care use (prior-year mental health treatment and prior-year substance abuse treatment). The missing data rate was less than 10% for all variables. We used multiple imputation methods to account for missing covariates (16).
Analysis
Within each group, we calculated unadjusted percentages of individuals receiving NMU medications from each source. To illustrate demographic, socioeconomic, health, and substance use differences across medication groups, we calculated means for the health and demographic variables stratified by NMU group. For all descriptive analyses, we conducted pairwise t tests to assess whether averages were different between the opioid analgesic NMU group and each of the other three NMU groups (tranquilizers or sedatives, stimulants, and multiple medications). For descriptive analyses, we focused on differences that are both likely to be clinically meaningful and statistically significant at the p<.01 level, applying a conservative p value threshold in order to account for multiple comparisons.
To evaluate the overall influence of these covariates on the probability of reporting a physician as the source of the NMU medication, we estimated logistic regression models for the probability that an individual received medication for NMU from a physician versus any nonphysician source (that is, friends and family or other sources). Models included sociodemographic factors, health insurance coverage, self-rated health status, substance use behaviors, criminal activity, and use of behavioral health care. We calculated variance inflation factors (VIFs) to test for multicollinearity. All VIFs were below the commonly accepted cutoff (<10) (17).
To illustrate subgroup differences shown by the regression models, we also provide predicted probabilities for receipt of opioid analgesics for NMU from each source (physician, friends-family, and other) for two hypothetical individuals: an older (>50), insured adult in fair-poor health who engaged in very frequent NMU (>60 days per year) and a younger (<26), uninsured individual who also used illicit drugs and engaged in moderate NMU (four to 13 days per year). These probabilities were derived by using predictive margins (18) from logistic regression models, with the dependent variable being the probability of having each source (physician, friends-family, and other) versus not having that source, with the same covariates described above.
For all estimates, we applied survey weights developed by NSDUH analysts to adjust for stratified sampling and nonresponse. We also adjusted standard errors to account for the sampling design and for the imputation methodology using the combined SVY and MI routines in Stata, version 13.1.
Results
The percentages of individuals across medication groups reporting different sources of NMU medication are reported in Table 1. Individuals using opioid analgesics were most likely to have a physician source (23.7%), double the percentages for individuals using tranquilizers-sedatives (11.9%) and stimulants (10.4%) and also higher than the percentage for those using multiple medications (20.1%). Friends or family was the most commonly reported source by all respondents, ranging from 67.8% for opioid analgesics to 84.2% for multiple medications (the combined percentages for the multiple-medication group exceeded 100% because individuals could report different sources for each medication). Obtaining medications for NMU from another source (that is, from a dealer, stole from a hospital or doctor’s office, bought on the Internet, wrote a fake prescription, or some other way) was reported by 8.5% of those in the opioid analgesic group; however, the rate of 16.0% for the multiple-medication group was significantly higher.
| Source | Opioid analgesics (N=18,219) | Tranquilizers-sedatives (N=3,651) | Stimulants (N=2,592) | >1 medication (N=10,228) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | p | N | % | p | N | % | p | |
| Physician | 4,017 | 23.7 | 427 | 11.9 | <.001 | 241 | 10.4 | <.001 | 1,618 | 20.1 | <.001 |
| Friends or family | 12,460 | 67.8 | 2,919 | 79.7 | <.001 | 2,106 | 78.1 | <.001 | 8,634 | 84.2 | <.001 |
| Other | 1,742 | 8.5 | 305 | 8.4 | .94 | 245 | 11.5 | .03 | 1,959 | 16.0 | <.001 |
TABLE 1. Reported sources of medications most recently obtained for nonmedical use (NMU) by 34,690 persons who reported prior-year NMUa
Table 2 presents data on selected characteristics for each group; analyses compared proportions for each group with the proportions for the opioid analgesic group. As shown, individuals in the stimulant and multiple-medication groups were younger than those in the opioid analgesic group, and individuals in the tranquilizer-sedative group tended to be older. Most individuals in the opioid analgesic and multiple-medication groups were male; however, in the tranquilizer-sedative and stimulant groups, most were female. For all groups, most respondents were non-Hispanic white. Low educational attainment and low income (<200% of the federal poverty level) were significantly more common in the opioid analgesic group than in the tranquilizer-sedative group.
| Characteristic | Opioid analgesics (N=18,219) | Tranquilizers-sedatives (N=3,651) | Stimulants (N=2,592) | >1 medication (N=10,228) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | p | N | % | p | N | % | p | |
| Demographic | |||||||||||
| Age | |||||||||||
| 12–25 | 14,022 | 39.7 | 2,395 | 27.4 | <.001 | 2,160 | 51.5 | <.001 | 8,146 | 46.9 | <.001 |
| 26–49 | 3,666 | 45.7 | 1,034 | 48.5 | .092 | 401 | 40.7 | .023 | 1,906 | 43.9 | .105 |
| >50 years | 531 | 14.6 | 222 | 24.1 | <.001 | 31 | 7.7 | <.001 | 176 | 9.1 | <.001 |
| Female | 8,672 | 44.1 | 2,227 | 60.3 | <.001 | 1,404 | 55.1 | <.001 | 5,107 | 47.0 | .007 |
| Race-ethnicity | |||||||||||
| Non-Hispanic white | 11,784 | 68.8 | 2,685 | 81.0 | <.001 | 2,031 | 79.8 | <.001 | 7,952 | 80.3 | <.001 |
| Non-Hispanic black | 2,141 | 11.0 | 241 | 5.3 | <.001 | 117 | 4.7 | <.001 | 482 | 5.4 | <.001 |
| Non-Hispanic other | 1,587 | 5.6 | 243 | 2.6 | <.001 | 192 | 5.0 | .27 | 760 | 3.9 | <.001 |
| Hispanic | 2,707 | 14.6 | 482 | 11.1 | <.001 | 252 | 10.5 | <.001 | 1,034 | 10.4 | <.001 |
| Socioeconomic status | |||||||||||
| High school graduate or less | 7,338 | 43.6 | 1,267 | 33.8 | <.001 | 634 | 27.3 | <.001 | 4,290 | 42.8 | .439 |
| Income <200% of federal poverty level | 8,510 | 40.6 | 1,491 | 32.5 | <.001 | 1,072 | 40.0 | .733 | 4,634 | 41.0 | .688 |
| Insurance coverage | |||||||||||
| Private | 9,479 | 55.3 | 2,130 | 62.2 | <.001 | 1,833 | 68.1 | <.001 | 5,396 | 54.0 | .209 |
| Medicaid only | 3,442 | 12.7 | 523 | 9.0 | <.001 | 266 | 8.2 | <.001 | 1,665 | 12.2 | .470 |
| Medicare only or other | 289 | 3.9 | 88 | 5.6 | .035 | 31 | 2.9 | .227 | 116 | 2.3 | .002 |
| Dual Medicaid-Medicare | 146 | 1.5 | 29 | 1.2 | .416 | 9 | 1.0 | .357 | 73 | 1.2 | .282 |
| Uninsured | 4,863 | 26.6 | 881 | 22.0 | .002 | 453 | 19.8 | <.001 | 2,978 | 30.3 | <.001 |
| Health behavior and substance use | |||||||||||
| Fair-poor health status | 1,694 | 12.6 | 337 | 11.8 | .563 | 136 | 7.4 | <.001 | 1,072 | 12.9 | .725 |
| Prior-year substance abuse treatment | 1,104 | 5.8 | 216 | 5.6 | .763 | 162 | 5.3 | .54 | 1,392 | 13.0 | <.001 |
| Prior-year mental health treatment | 3,514 | 20.5 | 1,098 | 33.0 | <.001 | 573 | 22.9 | .089 | 3,046 | 34.5 | <.001 |
| Sold drugs in prior year | 1,728 | 7.4 | 266 | 4.5 | <.001 | 259 | 9.1 | .094 | 2,484 | 21.0 | <.001 |
| Probation or parole in prior year | 1,579 | 7.0 | 264 | 5.4 | .044 | 201 | 8.5 | .249 | 1,502 | 12.8 | <.001 |
| Use of any illicit drugs in prior year | 9,977 | 45.3 | 1,981 | 41.9 | .039 | 1,792 | 62.2 | <.001 | 8,669 | 76.8 | <.001 |
| Days of NMU in prior year | |||||||||||
| ≤4 | 6,954 | 35.8 | 1,940 | 48.4 | <.001 | 1,141 | 41.2 | .006 | 1,289 | 12.4 | <.001 |
| 5–13 | 4,071 | 23.6 | 777 | 23.5 | .948 | 590 | 21.6 | .216 | 1,985 | 19.3 | <.001 |
| 14–59 | 4,232 | 23.3 | 601 | 17.2 | <.001 | 489 | 17.6 | <.001 | 2,911 | 28.6 | <.001 |
| ≥60 | 2,962 | 17.3 | 333 | 11.0 | <.001 | 372 | 19.5 | .12 | 4,043 | 39.7 | <.001 |
TABLE 2. Characteristics of 34,690 persons who reported prior-year nonmedical use (NMU) of selected prescription medicationsa
Fair-poor health was self-reported at similar rates in the opioid analgesic, tranquilizer-sedative, and multiple-medication groups; however, the proportion was lower in the stimulant group, compared with the opioid group. Compared with the opioid group, receipt of past-year substance abuse treatment was higher in the multiple-medication group, and receipt of mental health treatment was higher in the tranquilizer-stimulant and multiple-medication group. Illicit drug use was highest in the stimulant and multiple-medication groups. Selling drugs and being on probation or parole were most frequently reported by the multiple-medication group. Infrequent NMU (less than four days per year) was more common in the tranquilizer-sedative and stimulant groups, compared with the opioid analgesic group. Very frequent NMU (>60 days per year) was most common in the multiple-medication group. In a separate calculation (not shown), those with NMU for >60 days per year were estimated to account for 78% of all days of NMU across groups, indicating that even though this group was not the largest, they probably accounted for a very high volume of medication consumed.
Table 3 presents logistic regression models of the association between sociodemographic and other characteristics with obtaining medications for NMU from a physician versus obtaining them from a nonphysician source. In the tranquilizer-sedative and stimulant groups, there was no consistent relationship between age and probability of having a physician source. In the multiple-medication group, odds of having a physician source were higher for the older age group. Females tended to have lower odds of obtaining medication for NMU from physicians, but the relationship was significant only for opioid analgesics and tranquilizers-sedatives. Across groups, non-Hispanic blacks had greater odds of obtaining NMU medication from physician sources, and Hispanic and non-Hispanic “other” race individuals had significantly greater odds of having a physician source for opioids and tranquilizers. By contrast, educational attainment and income were not significant predictors of having a physician source. No significant differences were found between the privately insured and Medicaid-insured groups, but those with Medicare only or other insurance had increased odds of obtaining opioid analgesics and multiple medications for NMU from physician sources. Compared with privately insured persons, those dually covered by Medicare-Medicaid had significantly higher odds of having a physician source for opioid analgesics, tranquilizers-sedatives, and multiple medications. The uninsured had significantly lower odds of having a physician source for all medication groups except stimulants.
| Characteristic | Opioid analgesics | Tranquilizers-sedatives | Stimulants | >1 medication | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Demographic | ||||||||
| Age (reference: 12–25) | ||||||||
| 26–49 | .86** | .78–.94 | .91 | .71–1.17 | 1.11 | .78–1.57 | 1.38*** | 1.2–1.58 |
| >50 | 1.13 | .92–1.38 | 1.06 | .68–1.66 | 1.09 | .37–3.24 | 1.53* | 1.08–2.19 |
| Female | .86*** | .80–.93 | .73** | .58–.92 | .90 | .68–1.20 | .89 | .80–1.00 |
| Race-ethnicity (reference: Non-Hispanic white) | ||||||||
| Non-Hispanic black | 1.46*** | 1.32–1.65 | 2.06*** | 1.40–3.02 | 2.31*** | 1.38–3.85 | 1.54*** | 1.22–1.94 |
| Non-Hispanic other | 1.33*** | 1.18–1.52 | 1.52*** | 1.01–2.31 | 1.06 | .62–1.83 | .99 | .80–1.23 |
| Hispanic | 1.43*** | 1.29–1.58 | 1.82*** | 1.35–2.46 | 1.20 | .77–1.87 | 1.09 | .91–1.31 |
| Socioeconomic status | ||||||||
| High school graduate or less (reference: more than high school) | .93 | .86–1.01 | .85 | .67–1.09 | .85 | .61–1.20 | .89 | .79–1.01 |
| Income <200% of federal poverty level (reference: ≥200%) | 1.06 | .98–1.15 | .99 | .77–1.28 | .75 | .56–1.02 | .99 | .88–1.12 |
| Insurance (reference: private) | ||||||||
| Medicaid only | 1.11 | 1.00–1.23 | .82 | .59–1.16 | 1.34 | .87–2.09 | .99 | .84–1.16 |
| Medicare only or other | 1.32* | 1.01–1.72 | 1.28 | .70–2.32 | 1.61 | .59–4.41 | 2.01*** | 1.33–3.03 |
| Dual Medicaid-Medicare | 2.57*** | 1.82–3.64 | 4.12** | 1.73–9.82 | .62 | .07–5.40 | 1.84* | 1.11–3.06 |
| Uninsured | .77*** | .70–.85 | .68* | .50–.93 | 1.12 | .76–1.65 | .74*** | .64–.85 |
| Health behavior and substance use | ||||||||
| Fair-poor health status (reference: good or excellent) | 1.21** | 1.07–1.36 | 2.11*** | 1.54–2.88 | 2.10** | 1.26–3.47 | 1.38*** | 1.17–1.63 |
| Prior-year substance abuse treatment (reference: none) | .90 | .76–1.06 | 1.22 | .78–1.92 | 1.39 | .82–2.35 | 1.06 | .90–1.24 |
| Prior-year mental health treatment (reference: none) | 1.18*** | 1.08–1.30 | 2.20*** | 1.75–2.76 | 1.67** | 1.21–2.29 | 1.91*** | 1.69–2.16 |
| Sold drugs in prior year (reference: no) | .76*** | .65–.88 | .41** | .23–.71 | 1.16 | .70–1.90 | .79** | .68–.91 |
| Probation or parole in prior year (reference: no) | .93 | .81–1.07 | .49** | .29–.83 | 1.69* | 1.06–2.71 | .99 | .84–1.17 |
| Use of any illicit drugs in prior year (reference: none) | .53*** | .49–.57 | .71** | .56–.89 | .40*** | .30–.54 | .55*** | .47–.63 |
| Days of NMU in prior year (reference: ≤4) | ||||||||
| 5–13 | 1.25*** | 1.13–1.38 | 1.79*** | 1.35–2.37 | 1.25 | .85–1.85 | 1.22 | .97–1.54 |
| 14–59 | 1.59*** | 1.44–1.75 | 2.26*** | 1.68–3.03 | 2.13*** | 1.48–3.08 | 1.69*** | 1.36–2.09 |
| >60 | 1.89*** | 1.70–2.10 | 4.84*** | 3.50–6.70 | 2.38*** | 1.62–3.5 | 2.39*** | 1.94–2.93 |
TABLE 3. Logistic regression models of predictors of having a physician source (versus any nonphysician source) for medications used nonmedically among 34,690 persons who reported prior-year nonmedical use (NMU)a
Across groups, those with fair-poor self-rated health status had significantly higher odds of having a physician source for NMU medications. Prior-year substance abuse treatment receipt was not associated with having a physician source, but receipt of prior-year mental health treatment was associated with significantly higher odds of having a physician source across all groups. Individuals who reported selling drugs in the prior year had significantly lower odds of having a physician source for all medication groups except stimulants. Individuals on probation or parole had significantly lower odds of having a physician source for tranquilizers-sedatives but higher odds for stimulants. Across groups, individuals who used illicit drugs had lower odds of having a physician source. Across groups, there was also a positive and increasing relationship between greater frequency of NMU and having a physician source.
As an illustration of absolute differences in medication source, Figure 1 displays predicted probabilities of having different sources for two hypothetical individuals engaging in NMU opioid analgesic use: an older, sicker individual who engages in frequent NMU and a younger, uninsured individual who uses illicit drugs and engages in NMU less frequently. The older individual would have a 47.7% predicted probability of having a physician source, compared with 19.6% for the younger individual. By comparison, the predicted probability of having friends-family as a source would be 42.2% for the older individual versus 69.1% for the younger individual.
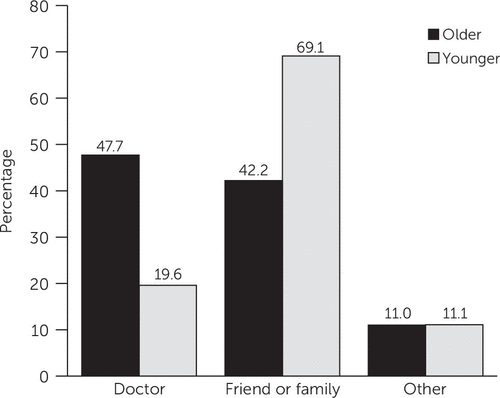
FIGURE 1. Predicted probabilities of receipt of medications for nonmedical use for two hypothetical individuals using opioid analgesics, by sourcea
aPredicted probabilities represent predictive margins from logistic regression models that examined the odds of receiving opioid analgesics from each source. The older individual is over age 50, insured, with fair-poor health and heavy nonmedical use (NMU) (>60 days per year). The younger individual is under age 26, uninsured, an illicit drug user, with moderate NMU (4–13 days per year).
Discussion
We compared physicians as a source for obtaining medications for NMU across medication groups. About one-quarter of individuals engaging in NMU of opioid analgesics obtained the medications from a physician. Rates of obtaining NMU medications from a physician were significantly lower for the tranquilizer-sedative, stimulant, and multiple-medication groups. Most individuals who engaged in NMU did not obtain medications from a physician; however, physicians were significantly more likely to be the source among individuals with the heaviest volume of NMU medications (>60 days per year). This group accounted for more than three-quarters of all days of NMU reported.
Characteristics associated with greater access to medical care increased the likelihood of obtaining medications for NMU from a physician source. This included insurance coverage, which may increase ability to pay. Use of mental health treatment significantly predicted having a physician as a source for NMU medications, suggesting the need for interventions to engage psychiatrists in addressing NMU among patients with mental illness. Individuals in worse health were also significantly more likely to receive NMU medications from physicians. These individuals are likely to come in more regular contact with medical care and may have chronic conditions that could lead physicians to prescribe medications, such as opioid analgesics for pain relief.
Our findings that persons from racial-ethnic minority groups were generally more likely to have obtained their medications from a physician source are surprising, because minority populations on average have lower access to and utilization of health care services than do non-Hispanic whites, even after adjustment for health and socioeconomic status (19,20). Thus they would be expected to have less access to a prescribing physician. Disentangling these differences and investigating the impact that prescription medication availability in social networks may play are important for future research.
Consistent with previous research, our study found that individuals with a higher frequency of NMU were more likely to have a physician source for all types of medication. One explanation is that such individuals may not be capable of obtaining desired quantities from social networks, requiring a source from which they can obtain larger quantities. By contrast, access to alternative sources for illicit substances could explain why individuals who reported use of illicit drugs were less likely to receive NMU medications from a physician. Closer engagement with street-drug trade may also explain why individuals who reported selling drugs were less likely to obtain NMU medications from a physician. It is unknown whether these individuals sold prescription medications specifically—and if so, what the source of supply for these medications might be.
Findings can inform current initiatives to reduce NMU of prescription medications. First, strategies to detect and reduce NMU in physician practices should be a priority, especially because individuals with the most frequent and problematic NMU were also more likely to obtain medications for NMU from a physician. Although two recent randomized controlled trials of Screening, Brief Intervention, and Referral to Treatment did not find this intervention effective in reducing drug use (21,22), it remains essential to identify patients engaged in NMU who have severe substance use disorders and initiate treatment in primary or specialty care. Teaching physicians to have open and frank conversations about NMU can also elicit patients’ concerns and beliefs about medication (for example, worry about chronic pain leading to nonindicated opioid use) that can help guide treatment planning and prompt conversations about treatment alternatives (23). Periodic urine toxicology testing for patients prescribed controlled substances, which is recommended by guidelines (24,25), may detect NMU in clinical practice and may be particularly important for the vulnerable group that uses multiple medications.
Clinical strategies can be augmented by policy changes. Among individuals with NMU, those with insurance (particularly Medicare) were more likely to obtain medications from a physician. Insurers can implement programs to identify and reduce NMU. For example, Medicaid programs have increased their role in identifying individuals with potentially problematic patterns of use and then enacting “lock-in” programs restricting these individuals to qualified pharmacies and prescribers (8). Prescription drug–monitoring programs may also enhance surveillance and detection efforts. Finally, because NMU may arise through medical use among patients with underlying health issues, such as pain or anxiety, efforts by payers to promote robust alternatives to prescription medications (such as acupuncture for pain management) could be an effective strategy (26).
This study had some limitations. First, the NSDUH measure of NMU is open to interpretation as to when individuals are taking medications beyond their prescribed purpose. Therefore, the measure could undercount some NMU, such as taking more medication than a physician recommends (27). Second, self-reported measures of NMU and other sensitive behaviors are subject to social desirability bias. The use of computer-assisted interviewing reduces bias, but greater validation of the NMU measures is needed (28). Third, NSDUH does not provide information about the specialty, practice setting, or demographic characteristics of the physician prescribing medications obtained for NMU, and NSDUH also lacks information about the clinical encounter (for example, whether physicians asked about possible misuse in the visit). Greater information about contact with physicians could be useful in identifying physicians who may be prescribing medications that are used nonmedically. Fourth, the public-use NSDUH data do not include geographic identifiers, which could be informative about the impacts of state-level policies to reduce NMU.
Conclusions
Our study provides insights into the characteristics of individuals who obtain medications for NMU from physicians versus other sources. Current initiatives such as prescription drug–monitoring programs and efforts to promote routine urine toxicology among persons prescribed controlled substances may help in reducing NMU but may only identify a small subset of people engaged in NMU—those who obtain medications from a physician. Because patients who reported more frequent NMU, received mental health treatment, and reported poor health were more likely to have obtained NMU medications from a physician, additional tools to identify, prevent, and manage NMU among medical patients are urgently needed.
1 Substance Use and Mental Health Estimates From the 2013 National Survey on Drug Use and Health: Overview of Findings. Rockville, Md, Substance Abuse and Mental Health Services Administration, 2014Google Scholar
2 : Subtypes of nonmedical prescription drug misuse. Drug and Alcohol Dependence 102:63–70, 2009Crossref, Medline, Google Scholar
3 : The history of barbiturates a century after their clinical introduction. Neuropsychiatric Disease and Treatment 1:329–343, 2005Medline, Google Scholar
4 : Toxicity and adverse consequences of benzodiazepine use. Psychiatric Annals 25:158–165, 1995Crossref, Google Scholar
5 : Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. Journal of the American Academy of Child and Adolescent Psychiatry 47:21–31, 2008Crossref, Medline, Google Scholar
6 : Association between use of benzodiazepines and risk of fractures: a meta-analysis. Osteoporosis International 25:105–120, 2014Crossref, Medline, Google Scholar
7 : Psychoactive substance use and the risk of motor vehicle accidents. Accident; Analysis and Prevention 36:631–636, 2004Crossref, Medline, Google Scholar
8 : Prescription opioid abuse: challenges and opportunities for payers. American Journal of Managed Care 19:295–302, 2013Medline, Google Scholar
9 : Transforming mental health care at the interface with general medicine: report for the President’s Commission. Psychiatric Services 57:37–47, 2006Link, Google Scholar
10 : Sources of prescription opioid pain relievers by frequency of past-year nonmedical use—United States, 2008–2011. JAMA Internal Medicine 174:802–803, 2014Crossref, Medline, Google Scholar
11 : Nonmedical use of prescription stimulants during college: four-year trends in exposure opportunity, use, motives, and sources. Journal of American College Health 60:226–234, 2012Crossref, Medline, Google Scholar
12 : Medical and nonmedical use of prescription drugs among secondary school students. Journal of Adolescent Health 40:76–83, 2007Crossref, Medline, Google Scholar
13 : Sources of prescriptions for misuse by adolescents: differences in sex, ethnicity, and severity of misuse in a population-based study. Journal of the American Academy of Child and Adolescent Psychiatry 48:828–836, 2009Crossref, Medline, Google Scholar
14 National Survey on Drug Use and Health, 2012. Rockville, Md, Substance Abuse and Mental Health Services Administration, 2013Google Scholar
15 Results From the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, Md, Substance Abuse and Mental Health Services Administration, 2015Google Scholar
16 : Multiple Imputation for Nonresponse in Surveys. New York, Wiley, 2004Google Scholar
17 : Multiple Regression: A Primer. Newbury Park, Calif, Pine Forge Press, 1999Google Scholar
18 : Predictive margins with survey data. Biometrics 55:652–659, 1999Crossref, Medline, Google Scholar
19 : Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatric Services 59:1264–1272, 2008Link, Google Scholar
20 : Racial/ethnic disparities in health: the interplay between discrimination and socioeconomic status. Ethnicity and Disease 9:151–165, 1998Google Scholar
21 : Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA 312:502–513, 2014Crossref, Medline, Google Scholar
22 : Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA 312:492–501, 2014Crossref, Medline, Google Scholar
23 : Opioid Prescribing for Chronic Pain—Achieving the Right Balance Through Education. New England Journal of Medicine 374:301–303, 2016Crossref, Medline, Google Scholar
24 : Diagnosis and management of adult attention-deficit/hyperactivity disorder. American Family Physician 85:890–896, 2012Medline, Google Scholar
25 : Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Annals of Internal Medicine 160:38–47, 2014Medline, Google Scholar
26 : Opioids, chronic pain, and addiction in primary care. Journal of Pain 11:1442–1450, 2010Crossref, Medline, Google Scholar
27 : Nonmedical use of prescription opioids: motive and ubiquity issues. Journal of Pain 9:473–486, 2008Crossref, Medline, Google Scholar
28 Reliability of Key Measures in the National Survey on Drug Use and Health. Rockville, Md, Substance Abuse and Mental Health Services Administration, 2010Google Scholar



