Efficacy of Sertraline in Preventing Relapse of Posttraumatic Stress Disorder: Results of a 28-Week Double-Blind, Placebo-Controlled Study
Abstract
OBJECTIVE: The study examined the efficacy of sertraline, compared with placebo, in sustaining improvement and preventing relapse over 28 weeks in patients with posttraumatic stress disorder (PTSD) who had completed a 12-week double-blind, placebo-controlled acute treatment study and a subsequent 24-week open-label study of continuation treatment with sertraline. METHOD: Ninety-six patients were randomly assigned, in a double-blind design, to 28 weeks of maintenance treatment with sertraline (50–200 mg, N=46; 78% were women) or placebo (N=50; 62% were women). Measures used in biweekly assessments included the Clinician-Administered PTSD Scale, the Impact of Event Scale, and the Clinical Global Impression severity and improvement ratings. Kaplan-Meier analyses were used to estimate time to discontinuation from the study due to relapse, relapse or study discontinuation due to clinical deterioration, and acute exacerbation. RESULTS: Continued treatment with sertraline yielded lower PTSD relapse rates than placebo (5% versus 26%). Patients who received placebo were 6.4 times as likely to experience relapse as were patients who received sertraline. Kaplan-Meier analyses confirmed the protective effect of sertraline in significantly extending time in remission. The ability of sertraline to sustain improvement was comparable across the three core PTSD symptom clusters (reexperiencing/intrusion, avoidance/numbing, and hyperarousal). A regression analysis found early response during acute treatment to be associated with a more than 16-fold reduced risk of relapse after placebo substitution. Sertraline, at a mean endpoint dose of 137 mg, was well tolerated, with no sertraline-related adverse events observed at a rate of 10% or higher. CONCLUSIONS: The results provide evidence for the ability of sertraline both to sustain improvement in PTSD symptoms and to provide prophylactic protection against relapse.
Posttraumatic stress disorder (PTSD) typically is a chronic illness, with a median time to recovery in the range of 3–5 years (1, 2). In one large community survey, 53% of patients with PTSD remained ill at 5 years, and fully 40% were still ill after 10 years, despite varying levels of naturalistic treatment in the community (2). PTSD is associated with high levels of health-related problems and disability and with impaired quality of life (3–9).
Over the past decade, research on acute treatments for PTSD has increased significantly. These studies generally have had small sample sizes and have been uncontrolled. The results have provided initial evidence for the efficacy of both cognitive behavior therapies (10–15) and antidepressants such as tricyclics (16, 17), monoamine oxidase inhibitors (16), and selective serotonin reuptake inhibitors in the acute treatment of PTSD (18–22). Two large placebo-controlled acute treatment studies have demonstrated the efficacy of sertraline in the treatment of PTSD (23, 24).
Anxiety and depressive disorders are generally chronic and/or recurrent and require long-term treatment (25–29). Studies have shown that continuation and maintenance medication reduce the likelihood of relapse or recurrence in depression (30), obsessive-compulsive disorder (OCD) (31), panic disorder (32), and social phobia (33). To our knowledge, no such studies exist for PTSD, leaving unanswered the important question whether maintenance therapy in PTSD protects patients from relapse or clinical deterioration.
The current article reports on the final phase of a series of studies that were conducted to provide data on the efficacy and tolerability of sertraline across the acute, continuation, and maintenance phases of PTSD treatment. In addition to the two acute sertraline treatment studies already cited (23, 24), another study (34) has examined and found additional clinical improvement over 6 months of open-label continuation treatment in patients who had been randomly assigned to receive sertraline and had completed 12 weeks of acute treatment for PTSD in a double-blind study. Ninety-two percent of the patients who met the criteria for response to sertraline during acute treatment sustained their response throughout 6 months of continuation therapy, while 58% of the patients who had not responded acutely converted to responder status during continuation treatment. Among responders in the acute phase, residual PTSD symptoms continued to show improvement.
We now report what is to our knowledge the first double-blind, placebo-controlled study designed to evaluate the efficacy of sertraline for relapse prevention in patients with PTSD who completed 6 months of open-label continuation sertraline treatment as responders.
Method
This article reports the results from a 28-week double-blind, placebo-controlled study of the safety, tolerability, and relapse prevention efficacy of maintenance treatment with sertraline compared to placebo in PTSD conducted at 24 centers in the United States. Patients who were eligible to enter the study were completers in two previous studies: first, one of two acute double-blind treatment studies with an identical design in which the patients were randomly assigned to receive sertraline or placebo; and second, a 24-week open-label continuation study that completers from the acute treatment trials (regardless of response status) were eligible to enter within 3 days of their last acute-phase visit. To preserve the double blind from the acute treatment study, all patients were discontinued from their acute-phase study medication (sertraline or placebo) before initiating open-label study treatment with a daily dose of 25 mg of sertraline. At the end of the first week, the daily dose of sertraline was increased to 50 mg. Dosing throughout the open-label phase was flexible, in the range of 50 mg to 200 mg, and was based on clinical response and tolerability. The results of the study of continuation treatment with sertraline have previously been reported (34).
Study Patients
The enrollees in the current 28-week double-blind study were male and female outpatients at least 18 years of age who had completed the previous 24 weeks of open-label continuation treatment with sertraline and who met responder criteria at the final two visits. The responder criteria were a Clinical Global Impression improvement score ≤2 (much or very much improved) (35) and ≥30% improvement in the total severity score in part 2 of the Clinician-Administered PTSD Scale (36), both indexed against the pretreatment baseline of the original double-blind acute treatment study. Additional entry criteria required that the patients have no clinically significant abnormalities identified in a physical examination and laboratory testing conducted at the end of week 24 of continuation treatment study and that female patients use medically acceptable birth control throughout the study.
The original eligibility criteria that defined the study group have been presented in detail in a previous publication (23). Briefly, at baseline of the two acute treatment studies, the patients met DSM-III-R criteria for a principal diagnosis of PTSD as determined by part 1 of the Clinician-Administered PTSD Scale (37). A minimum 6-month duration of PTSD symptoms was required, as well as a total severity score ≥50 on part 2 of the Clinician Administered PTSD Scale at the end of a 2-week placebo run-in period; subjects were thus at least moderately ill. Subjects were excluded if they had a current or past history of bipolar disorder, schizophrenia, or organic mental disorder; a primary diagnosis of major depression, OCD, or other anxiety disorders; or alcohol or other substance dependence or abuse in the past 6 months, or if they met other exclusion criteria summarized in a previous report (23).
Concomitant psychotropic therapy, with the exception of chloral hydrate for sleep (not more than two nights per week), was prohibited during all studies in the series. Cognitive behavior therapy was not permitted during any of the trials, and other forms of psychotherapy could not be initiated or ended during a trial.
The current study was approved by the institutional review board at each of the 24 collaborating centers or by a national institutional review board. After a complete description of the study to the subjects, written informed consent was obtained.
Efficacy Measures
Patients were evaluated and rated biweekly during the 28 weeks of double-blind treatment. The two a priori primary outcomes (and the related time-to-event measures) were 1) rate of relapse (and time before relapse) and 2) rate of relapse or discontinuation from the study due to clinical deterioration (and time before relapse or time before clinical deterioration).
A patient was classified as relapsed (and therefore was discontinued from the study) if he or she met all of the following three criteria on two consecutive visits: 1) CGI improvement score ≥3 (relative to the baseline of the original acute treatment study), 2) an increase in the Clinician-Administered PTSD Scale, part 2, score by ≥30% compared to the baseline of the current double-blind study and an increase of ≥15 points on the Clinician-Administered PTSD Scale, part 2, score, and 3) in the investigator’s opinion, significant worsening of the patient’s clinical condition.
Acute exacerbation was a third outcome that was intended to refer to patients whose symptoms worsened during the course of the study, regardless of whether they met relapse criteria or were discontinued from the study because of clinical deterioration.
The secondary outcome measures for the study consisted of 1) the 17-item total severity score on part 2 of the Clinician-Administered PTSD Scale (36, 37), a 30-item investigator-completed scale that rates both the frequency and intensity of PTSD symptoms on separate 5-point scales, 2) score on the Impact of Event Scale (38, 39), a 15-item patient-completed scale that rates intrusion and avoidance symptoms on a 4-point severity scale, and 3) the investigator-rated CGI severity and improvement scale scores (35). Change in score on the CGI improvement scale was assessed with reference to the pretreatment baseline of the acute treatment studies. Secondary outcome measures also included 4) the score on the patient-rated 17-item Davidson Trauma Scale (40, 41), which rates the 17 DSM-III-R-defined PTSD symptoms on a 5-point frequency and a 5-point severity scale, 5) score on the investigator-rated 24-item Hamilton Depression Rating Scale (42), 6) score on the short form of the validated patient-rated Quality of Life Enjoyment and Satisfaction scale (43), 7) scores on subscales of part 2 of the Clinician-Administered PTSD Scale, the Impact of Event Scale, and the Davidson Trauma Scale that report the severity of the three PTSD symptom clusters (reexperiencing/intrusion, avoidance/numbing, and hyperarousal), and 8) scores on the subscales of the Clinician-Administered PTSD Scale, part 2, that measure associated features and functional impairment. The Davidson Trauma Scale was completed by patients at every assessment visit. The Hamilton depression scale and the Quality of Life Enjoyment and Satisfaction scale were completed at baseline in the 28-week maintenance study and at week 28 or at study discontinuation if that occurred before week 28.
Safety Assessments
At each study visit, patients’ weight, sitting blood pressure, and heart rate were assessed. Data recorded on side effects that were observed or spontaneously reported included time of onset, duration, severity, action taken, and outcome. Data on concomitant medications included the daily dose, stop and start dates, and the reason for use. A physical examination, a 12-lead ECG, and laboratory assessments (e.g., clinical chemistry, hematology, and urinalysis) were performed at the maintenance phase baseline and at week 28 (or at the time of study discontinuation).
Compliance was monitored by counts of returned medication, and patients who were found to be noncompliant were counseled. Overall, four patients met criteria for noncompliance (≥4 consecutive days of missed doses), but only at one study visit. Data for these four patients were included in the efficacy analysis.
Statistical Methods
Baseline characteristics of each treatment group were compared with chi-square for tests for proportions for categorical data and two-way analysis of variance models for continuous variables, with treatment and site as main effects.
The main purpose of this study was to compare rates (and times to event) for the following three outcomes: 1) relapse, 2) relapse or discontinuation because of clinical deterioration (self-rated), and 3) acute exacerbation of PTSD. The proportion of patients in each treatment group who experienced one of the three outcome events (relapse, relapse or discontinuation because of clinical deterioration, or acute exacerbation) were compared by using Fisher’s exact test. Odds ratios with 95% confidence intervals were calculated for each outcome. Kaplan-Meier estimates were used to assess time until discontinuation because of relapse, relapse or discontinuation because of clinical deterioration, and acute exacerbation. The Kaplan-Meier estimates for the two treatment groups were compared by using a log-rank test.
For secondary outcome measures, treatment group comparisons were based on change from baseline to endpoint (except for the CGI improvement scale score, which was measured as improvement from baseline) and were performed by using analysis of covariance models with main effects for treatment and site and baseline score as a covariate. Logistic regression models were used to explore which factors could predict relapse, backward selection methods were used to identify those predictors that significantly differentiated patients with a relapse from those with no relapse. Odds ratios and 95% confidence intervals were estimated on the basis of the model results.
All hypothesis tests were performed with the type III sums of squares from the SAS GLM (general linear models) procedure (SAS, Cary, N.C.). All statistical analyses were two-sided with a significance level set at 0.05. Adjustments for multiple comparisons were not made. Such adjustments were judged to be overly conservative given that repeated comparisons were not made within the same hypothesis (and when they were, as in the logistic regression, the model adjusted for these comparisons).
Results
Patients’ Characteristics
Ninety-six patients entered the 28-week double-blind treatment study and were randomly assigned to receive either sertraline or placebo. Figure 1 summarizes the participation in previous treatment studies of the patients who participated in the current study. The patients were predominantly female (sertraline group: N=36, or 78%; placebo group: N=31, or 62%) (χ2=3.01, df=1, p=0.08) and ranged in age from 21 to 69 years (sertraline group: mean age=44.9 years, SD=9.8; placebo group: mean age=42.0, SD=10.8) (F=2.01, df=1, 76, p=0.16). The mean duration of illness was 12.2 years (SD=13.0) in the sertraline treatment group and 13.9 years (SD=12.8) in the placebo treatment group (F=0.42, df=1, 76, p=0.52). Of the 96 patients who entered the study, 38 (39.6%) currently met criteria for a secondary depressive disorder and 19 (19.8%) met criteria for a secondary anxiety disorder.
The distribution of index traumatic events, defined as the event that was most distressing to the patient, was as follows for the sertraline and placebo treatment groups, respectively: serious accident, injury, or fire: N=3 (6.5%) and N=3 (6.0%); physical or sexual assault: N=24 (52.2%) and N=29 (58.0%); seeing someone hurt or die: N=7 (15.2%) and N=5 (10.0%); being in a war or combat: N=4 (8.7%) and N=5 (10.0%); and miscellaneous other events: N=8 (17.4%) and N=8 (16.0%).
Measures of Relapse Prevention
Sertraline (N=38) demonstrated a significant advantage over placebo (N=46) in prevention of PTSD relapse on three outcome criteria: 1) relapse (sertraline: N=2 or 5.3%; placebo: N=12 or 26.1%) (p<0.02, Fisher’s exact test), 2) relapse or discontinuation due to clinical deterioration (sertraline: N=6 or 15.8%; placebo: N=21 or 45.7%) (p=0.005, Fisher’s exact test), and 3) acute exacerbation of PTSD symptoms (sertraline: N=6 or 15.8%; placebo: N=24 or 52.2%) (Fisher’s exact test, p<0.001). An analysis of outcome by gender showed that sertraline had clear relapse prevention efficacy in both male and female patients. None of the nine male patients treated with sertraline suffered a PTSD relapse, compared with 27.8% of male patients who received placebo (N=5 of 18) (p=0.14, Fisher’s exact test); for female patients, the PTSD relapse rates were 6.9% for those treated with sertraline (N=2 of 29) and 25.0% for those who received placebo (N=7 of 28) (p=0.08, Fisher’s exact test). Similar results were found for the other two outcome measures. Only 11.1% of the male patients treated with sertraline (N=1 of 9), compared to 44.4% of the male patients who received placebo (N=8 of 18), either relapsed or discontinued participation in the study because of clinical deterioration (p=0.19, Fisher’s exact test). Among female patients, 17.2% of those treated with sertraline (N=5 of 29) and 46.4% of those who received placebo (N=13 of 28) either relapsed or discontinued because of clinical deterioration (p<0.03, Fisher’s exact test). An acute exacerbation of PTSD symptoms was experienced by 11.1% of the male patients treated with sertraline (N=1 of 9), compared with 50.0% of the male patients who received placebo (N=9 of 18) (p=0.10, Fisher’s exact test), and by 17.2% of the female patients treated with sertraline (N=5 of 29), compared with 56.3% of the female patients who received placebo (N=15 of 28) (N=0.006, Fisher’s exact test). The results for male patients must be viewed as preliminary, given the small number of male patients in the two treatment groups (N=29).
The adjusted relative risk of placebo treatment (i.e., discontinuing sertraline treatment) was estimated under the assumption of proportional hazard rates for the three outcome criteria. Patients receiving placebo were 6.35 times as likely (95% CI=1.32–30.49) to experience relapse of PTSD as were patients receiving sertraline. Patients receiving placebo were 4.48 times as likely (95% CI=1.57–12.77) to experience relapse or discontinuation due to clinical deterioration as were patients receiving sertraline. Patients receiving placebo were 5.82 times as likely (95% CI=2.04–16.57) to experience an acute exacerbation of PTSD symptoms as were patients receiving sertraline.
Figure 2 shows the Kaplan-Meier estimates of the cumulative time-to-relapse probabilities for two of the three outcome criteria: discontinuation due to relapse or clinical deterioration and acute exacerbation of PTSD symptoms. Both showed a highly significant relapse prevention advantage for sertraline compared with placebo (discontinuation due to relapse or clinical deterioration: log-rank test, χ2=9.98, df=1, p=0.002; acute exacerbation: log-rank test, χ2=13.01, df=1, p<0.001). The third clinical outcome criteria, time to full PTSD relapse, showed a similar significant advantage in favor of sertraline (log-rank test, χ2=7.26, df=1, p=0.007).
Measures of Symptom Severity
The prophylactic efficacy advantage of sertraline was also reflected in symptom severity scores showing that sertraline-treated patients maintained the gains achieved during 24 weeks of open-label treatment, while patients who received placebo significantly worsened (Figure 3). The decrease in Clinician-Administered PTSD Scale, part 2, severity scores between weeks 4 and 6 appears to have been largely due to the fact that nine patients who received placebo discontinued participation in the study during this 2-week period because of clinical worsening (one-third of the overall attrition observed among patients receiving placebo). In a last-observation-carried-forward endpoint analysis, total scores on the Davidson Trauma Scale, the Impact of Event Scale, and the CGI severity scale also showed minimal change during the maintenance phase of the study in patients treated with sertraline and significantly greater worsening in patients treated with placebo. The endpoint change scores (least squares means and standard errors) for the sertraline group and the placebo group, respectively, were 1.1 (SE=1.6) versus 5.9 (SE=1.5) (F=5.06, df=1, 64, p<0.03) on the Impact of Event Scale, 6.7 (SE=3.6) versus 19.9 (SE=3.4) (F=7.49, df=1, 63, p=0.008) on the Davidson Trauma Scale, and 0.2 (SE=0.2) versus 1.0 (SE=0.2) (F=10.25, df=1, 64, p=0.002) on the CGI severity scale.
Male and female patients maintained their gains similarly. Among patients treated with sertraline who did not meet any of the three outcome criteria indicating relapse, the mean change scores at study endpoint for part 2 of the Clinician-Administered PTSD Scale, Impact of Event Scale, and Davidson Trauma Scale were minimal (1.7 [SD=11.9], –1.1 [SD=5.2], and 0.8 [SD=13.3], respectively), indicating that these patients maintained their treatment gains.
Secondary Efficacy Measures
The efficacy advantage of sertraline was consistent across the three core symptom clusters of PTSD, as well as across the associated features measured on the Clinician-Administered PTSD Scale, part 2. Patients treated with placebo had worsened symptom severity at endpoint, compared to patients treated with sertraline, who maintained their treatment gains. The endpoint change scores on the Clinician-Administered PTSD Scale, part 2, for the sertraline group and the placebo group, respectively, were 0.6 (SD=0.9) versus 4.0 (SD=0.9) (F=7.76, df=1, 64, p=0.007) for the reexperiencing/intrusion cluster, 2.5 (SD=1.6) versus 7.3 (SD=1.5) (F=5.00, df=1, 64, p<0.03) for the avoidance/numbing cluster, and 1.3 (SD=1.4) versus 9.2 (SD=1.3) (F=17.51, df=1, 64, p<0.001) for the arousal cluster.
Patients receiving sertraline had only modest mean changes in their 24-item Hamilton depression scale total score from baseline to study endpoint (2.5 [SD=1.7], compared to 7.8 [SD=1.6] for patients receiving placebo) (F=5.60, df=1, 52, p=0.02).
Consistent with the results showing sustained improvement across all symptom severity measures, patients treated with sertraline maintained significantly more of the improvement in their quality of life than did patients treated with placebo. The endpoint Quality of Life Enjoyment and Satisfaction scale change score was –4.4 (SD=2.6) for patients treated with sertraline versus –13.7 (SD=2.7) for patients receiving placebo (F=6.45, df=1, 50, p=0.01).
An exploratory logistic regression analysis was performed in an attempt to identify patient characteristics that were associated with a higher risk of relapse. Candidate predictor variables included sex, age, type of trauma (interpersonal violence versus other), presence of axis I depression comorbidity, presence of residual PTSD symptoms (Clinician-Administered PTSD Scale, part 2, score at baseline in the maintenance treatment study), and early response status during the acute treatment study (CGI improvement score ≤2 by week 4). Early response during acute treatment was found to be a strong and highly significant predictor of sustained improvement (i.e., no relapse) (R2=0.32; χ2=17.50, df=8, p=0.03; with p=0.0009 when a backward selection procedure was used and all nonsignificant predictor variables were eliminated). The associated reduction in relapse risk due to early response was described by an odds ratio of 16.9 (95% CI=2.8–328.4). None of the early responder patients treated with sertraline in the acute phase relapsed, and only one early responder patient treated with placebo relapsed.
Tolerability and Patient Disposition
The mean endpoint daily dose for patients treated with sertraline was 137 mg/day (SD=52). The mean daily dose of placebo equivalent was 145 mg/day (SD=58).
Overall, 60.9% of the patients who received sertraline (N=28) and 40.0% of the patients who received placebo (N=20) completed the study. Reasons for study discontinuation for the patients who received sertraline and for those who received placebo, respectively, were: met relapse criteria, 6.5% (N=3) versus 28.0% (N=14); clinical deterioration, 10.9% (N=5) versus 20.0% (N=10); adverse events, 8.7% (N=4) versus 6.0% (N=3); withdrew consent, 6.5% (N=3) versus 6.0% (N=3); and miscellaneous other reasons, 6.5% (N=3) versus 0% (N=0). Six patients who received sertraline and four patients who received placebo at one study site were excluded from the efficacy analyses because of the institutional review board’s concerns about a study investigator’s lack of participation in performing the required efficacy assessments.
There were no treatment-emergent, treatment-related adverse events reported at a rate of 10% or higher for the sertraline-treated patients during the 28 weeks of double-blind sertraline treatment. The only treatment-related adverse event reported at a rate of 10% or higher for the placebo group was dizziness (18.0% [N=9] versus 4.3% [N=2] of the patients receiving sertraline) (p=0.053, Fisher’s exact test). At 2 weeks, 18% of the placebo subjects (N=9) and 4.3% of the sertraline subjects (N=2) experienced dizziness (χ2=4.07, df=1, p=0.04). Among the placebo subjects, three reported mild, five moderate, and one severe dizziness. Among the sertraline subjects, one reported mild and one moderate dizziness. This dizziness may have been a transient symptom related to rapid discontinuation of sertraline after 6 months or more of continuous treatment.
There were no clinically significant laboratory abnormalities during the study. Three of 44 patients who received sertraline (6.8%), and four of 50 patients who received placebo (8.0%) gained 7% or more in body weight during the 28 weeks of study treatment.
Discussion
The results of this double-blind, placebo-controlled maintenance treatment study found sertraline to have significant efficacy compared to placebo in sustaining improvement in symptom severity achieved during previous acute and continuation treatment and in preventing relapse. By all three a priori outcome criteria, sertraline showed a significant advantage over placebo in preventing PTSD relapse and was associated with a 4.5- to 6.4-fold reduction in the likelihood of a recurrence of PTSD. This prophylactic benefit was observed for both men and women, and it was notable that none of the men who received sertraline met relapse criteria during the 28-week study.
Kaplan-Meier analyses (Figure 2) demonstrated that sertraline significantly extends the time in remission among patients with chronic PTSD (mean duration=13 years). In most patients treated with sertraline, efficacy was well sustained, and only 5% of sertraline-treated patients met relapse criteria. Among patients randomly assigned to receive placebo, the largest proportion of clinical worsening occurred during the first 2 months after sertraline discontinuation, underscoring the need for increased observation of patients during the initial weeks after the end of continuation treatment. The possibility that discontinuation of sertraline after 36 weeks of treatment might have caused some mild discontinuation symptoms (the dizziness observed in patients who received placebo) needs to be considered as a factor that may have contributed to relapse during the early weeks of placebo treatment. The majority of cases of relapse, however, occurred 3 or more weeks after sertraline was discontinued, suggesting that loss of prophylactic efficacy was a significant factor. Furthermore, a recent systematic review of relapse after antidepressant discontinuation found that abrupt discontinuation did not increase the risk of relapse (44).
Overall, sertraline treatment was well tolerated, with a very low incidence of adverse events among patients receiving sertraline (all adverse events occurred at a rate lower than 10%). Consistent with the findings of previous long-term treatment studies (45), sertraline was not associated with significant weight gain, compared to placebo.
The efficacy of sertraline in maintaining earlier therapeutic gains was consistent across each of the three core PTSD symptom cluster measures, which showed only minimal endpoint symptom change scores.
Patients in the current study had previously completed 12 weeks of double-blind, placebo-controlled acute treatment, as well as 6 months of open-label continuation treatment. During the continuation phase of treatment, 92% of acute phase responders maintained their response, while 54% of acute phase nonresponders converted to responder status (the majority in the first 6 weeks). Among patients who maintained their response, there was a modest but significant further reduction in symptom severity during continuation treatment.
An exploratory regression analysis was able to identify only one predictor of sustained response among patients whose sertraline was discontinued: early response, in the first 4 weeks of acute treatment, was associated with a significantly reduced risk of relapse after placebo substitution. The fact that early responder status was a positive predictor regardless of whether the patient received sertraline or placebo in the initial acute treatment study suggests that early response, although significantly more frequent among patients who received sertraline, is actually a nonspecific prognostic factor associated with a highly favorable long-term clinical outcome.
Several limitations of this study should be considered. First, the study patients suffered from PTSD that was highly chronic (mean duration=13 years) and of moderate or greater severity. Consequently, it is unclear to what extent the results of this study generalize to the treatment of patients with less chronic or less severe PTSD. Furthermore, we excluded patients with several forms of current or lifetime psychopathology, including a primary diagnosis of major depression, OCD or other anxiety disorders, and alcohol or other substance dependence or abuse in the past 6 months. These exclusions may also affect the generalizability of the findings. Finally, the size of the study group was relatively small, suggesting the need for additional studies to cross-validate the results.
Future studies will be needed to address clinical questions such as when maintenance therapy is indicated, how long maintenance sertraline treatment should be continued, and the optimal dose of sertraline required both to sustain improvement and to prevent relapse.
The current study is the first double-blind, placebo-controlled treatment trial we are aware of that examines the efficacy and tolerability of long-term pharmacotherapy for PTSD. The results confirm the clinical efficacy and safety of sertraline in preventing PTSD relapse and sustaining response among responders. This is an important finding in light of the established chronicity of PTSD. The results of the current study are consistent with previous research suggesting the importance of maintenance treatment in the long-term management of other affective and anxiety disorders such as depression (30), OCD (31), social phobia (33), and panic disorder (32).
Earlier versions presented as posters at the 153rd annual meeting of the American Psychiatric Association, Chicago, May 13–18, 2000; a meeting of the Collegium Internationale Neuro-Psychopharmacologicum, Brussels, July 9–13, 2000; and a meeting of the European College of Neuropsychopharmacology, London, Sept. 21–25, 2000. Received Dec. 18, 2000; revision received June 29, 2001; accepted Aug. 11, 2001. From the Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; Butler Hospital and Brown University, Providence, R.I.; Seattle Clinical Research Center, Seattle; Medical University of South Carolina, Charleston; Emory University, Atlanta; University of Colorado School of Medicine, Denver; University of California, Davis; Dartmouth University, Dartmouth, N.H.; and Pfizer, Inc., New York. Address reprint requests to Dr. Davidson, Department of Psychiatry and Behavioral Sciences, Anxiety and Traumatic Stress Program, Duke University Medical Center, Box 3812, Durham, NC 27710; [email protected] (e-mail). Supported by a grant from Pfizer. This study was completed with the cooperation and participation of the following investigators: Gregory M. Asnis, M.D., Dewleen Baker, M.D., Robert Bielski, M.D., Susanna Goldstein, M.D., David Goldstein, M.D., Henry Lahmeyer, M.D., Murray Rosenthal, D.O., Ward Smith, M.D., Lynn Cunningham, M.D., Eugene DuBoff, M.D., Wayne Goodman, M.D., Jon F. Heiser, M.D., William Patterson, M.D., and Jeffery Rausch, M.D.
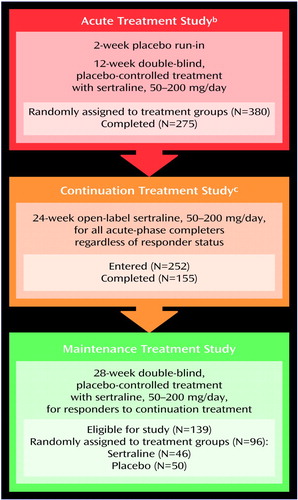
Figure 1. History of Participation in Treatment Studies of Patients With PTSD Recruited for a 28-Week Double-Blind, Placebo-Controlled Study of Maintenance Treatment With Sertralinea
aEligibility and exclusion criteria presented elsewhere (23). Eligibility criteria included a DSM-III-R diagnosis of PTSD, a minimum 6-month duration of PTSD symptoms, and a total severity score of ≥50 on part 2 of the Clinician-Administered PTSD Scale (36) at the end of the 2-week placebo run-in period. Exclusion criteria included a current or past history of bipolar disorder, schizophrenia, or organic mental disorder; a primary diagnosis of major depression, obsessive-compulsive disorder, or other anxiety disorder; or alcohol or other substance dependence or abuse in the past 6 months.
bResults reported elsewhere (23, 24).
cResults reported elsewhere (34).
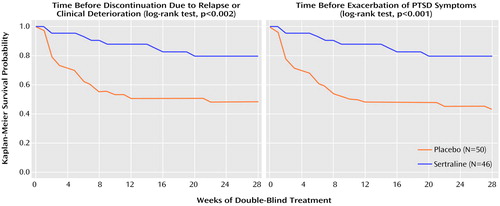
Figure 2. Kaplan-Meier Analysis of Time Before Discontinuation Due to Relapse or Clinical Deterioration and Time Before Exacerbation of PTSD Symptoms Among Patients in a 28-Week Double-Blind, Placebo-Controlled Study of Maintenance Treatment With Sertralinea
aPatients eligible to enter the maintenance study had participated in a 12-week double-blind, placebo-controlled study of acute treatment with sertraline and had responded to treatment with sertraline in a 24-week open-label continuation study. Sertraline dose during the maintenance treatment study was 50–200 mg/day.
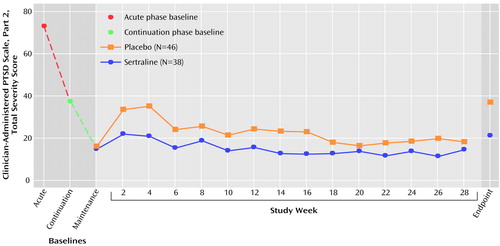
Figure 3. Mean Total Severity Score on the Clinician-Administered PTSD Scale, Part 2, at Baseline in Studies of Acute and Continuation Sertraline Treatment for PTSD and During a 28-Week Double-Blind, Placebo-Controlled Study of Maintenance Treatment With Sertralinea
aThe acute treatment study consisted of 12 weeks of double-blind, placebo-controlled treatment with sertraline, 50–200 mg/day. The continuation study consisted of 24 weeks of open-label treatment with sertraline, 50–200 mg/day.
1. Breslau N, Davis GC: Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry 1992; 149:671-675Link, Google Scholar
2. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048-1060Crossref, Medline, Google Scholar
3. Solomon SD, Davidson JR: Trauma: prevalence, impairment, service use, and cost. J Clin Psychiatry 1997; 58:5-11Crossref, Medline, Google Scholar
4. Friedman MJ, Schnurr PP: The relationship between trauma, posttraumatic stress disorder, and physical health, in Neurobiological and Clinical Consequences of Stress: From Normal Adaption to PTSD. Edited by Friedman MJ, Charney DS, Deutch AY. Philadelphia, Lippincott-Raven Press, 1995, pp 507-524Google Scholar
5. Kimerling R, Calhoun KS: Somatic symptoms, social support, and treatment-seeking among sexual assault victims. J Consult Clin Psychol 1994; 62:333-340Crossref, Medline, Google Scholar
6. Davidson JR, Hughes D, Blazer DG, George LK: Post-traumatic stress disorder in the community: an epidemiological study. Psychol Med 1991; 21:713-721Crossref, Medline, Google Scholar
7. Zatzick DF, Marmar CR, Weiss DS, Browner WS, Metzler TJ, Golding JM, Stewart A, Schlenger WE, Wells KB: Posttraumatic stress disorder and functioning and quality of life outcomes in a nationally representative sample of male Vietnam veterans. Am J Psychiatry 1997; 154:1690-1695Link, Google Scholar
8. Warshaw MG, Fierman E, Pratt L, Hunt M, Yonkers KA, Massion AO, Keller MB: Quality of life and dissociation in anxiety disorder patients with histories of trauma or PTSD. Am J Psychiatry 1993; 150:1512-1516Link, Google Scholar
9. Amaya-Jackson L, Davidson JR, Hughes DC, Swartz M, Reynolds V, George LK, Blazer DG: Functional impairment and utilization of services associated with posttraumatic stress in the community. J Trauma Stress 1999; 12:709-724Crossref, Medline, Google Scholar
10. Brom D, Kleber RJ, Defares PB: Brief psychotherapy for posttraumatic stress disorders. J Consult Clin Psychol 1989; 57:607-612Crossref, Medline, Google Scholar
11. Marks I, Lovell K, Noshirvani H, Livanou M, Thrasher S: Treatment of posttraumatic stress disorder by exposure and/or cognitive restructuring: a controlled study. Arch Gen Psychiatry 1998; 55:317-325Crossref, Medline, Google Scholar
12. Cahill SP, Carrigan MH, Frueh BC: Does EMDR work? And if so, why? a critical review of controlled outcome and dismantling research. J Anxiety Disord 1999; 13:5-33Crossref, Medline, Google Scholar
13. Shapiro F: Eye movement desensitization and reprocessing (EMDR): evaluation of controlled PTSD research. J Behav Ther Exp Psychiatry 1996; 27:209-218Crossref, Medline, Google Scholar
14. Boudewyns PA, Hyer L: Physiological response of combat memories and preliminary treatment outcome in Vietnam veteran PTSD patients treated with direct therapeutic exposure. Behav Ther 1990; 21:63-87Crossref, Google Scholar
15. Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP: A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol 1999; 67:194-200Crossref, Medline, Google Scholar
16. Kosten TR, Frank JB, Dan E, McDougle CJ, Giller EL Jr: Pharmacotherapy for posttraumatic stress disorder using phenelzine or imipramine. J Nerv Ment Dis 1991; 179:366-370Crossref, Medline, Google Scholar
17. Davidson J, Kudler H, Smith R, Mahorney SL, Lipper S, Hammett E, Saunders WB, Cavenar JO Jr: Treatment of posttraumatic stress disorder with amitriptyline and placebo. Arch Gen Psychiatry 1990; 47:259-266Crossref, Medline, Google Scholar
18. van der Kolk BA, Dreyfuss D, Michaels M, Shera D, Berkowitz R, Fisler R, Saxe G: Fluoxetine in posttraumatic stress disorder. J Clin Psychiatry 1994; 55:517-522Medline, Google Scholar
19. Connor KM, Sutherland SM, Tupler LA, Malik ML, Davidson JR: Fluoxetine in post-traumatic stress disorder: randomised, double-blind study. Br J Psychiatry 1999; 175:17-22Crossref, Medline, Google Scholar
20. Brady KT, Sonne SC, Roberts JM: Sertraline treatment of comorbid posttraumatic stress disorder and alcohol dependence. J Clin Psychiatry 1995; 56:502-505Medline, Google Scholar
21. Rothbaum BO, Ninan PT, Thomas L: Sertraline in the treatment of rape victims with posttraumatic stress disorder. J Trauma Stress 1996; 9:865-871Crossref, Medline, Google Scholar
22. Marshall RD, Schneier FR, Fallon BA, Knight CB, Abbate LA, Goetz D, Campeas R, Liebowitz MR: An open trial of paroxetine in patients with noncombat-related, chronic posttraumatic stress disorder. J Clin Psychopharmacol 1998; 18:10-18Crossref, Medline, Google Scholar
23. Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM: Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA 2000; 283:1837-1844Crossref, Medline, Google Scholar
24. Davidson JR, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM: Multi-center, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry 2001; 58:485-492Crossref, Medline, Google Scholar
25. Keller MB, Boland RJ: Implications of failing to achieve successful long-term maintenance treatment of recurrent unipolar major depression. Biol Psychiatry 1998; 44:348-360Crossref, Medline, Google Scholar
26. Yonkers KA, Warshaw MG, Massion AO, Keller MB: Phenomenology and course of generalised anxiety disorder. Br J Psychiatry 1996; 168:308-313Crossref, Medline, Google Scholar
27. Katschnig H, Amering M: The long-term course of panic disorder and its predictors. J Clin Psychopharmacol 1998; 18(6, suppl 2):6S-11SGoogle Scholar
28. Kessler RC, Stein MB, Berglund P: Social phobia subtypes in the National Comorbidity Survey. Am J Psychiatry 1998; 155:613-619Link, Google Scholar
29. Skoog G, Skoog I: A 40-year follow-up of patients with obsessive-compulsive disorder. Arch Gen Psychiatry 1999; 56:121-127Crossref, Medline, Google Scholar
30. Agency for Health Care Policy Research: Treatment of Major Depression: Clinical Guidelines, vol 5, number 2: AHCPR Publication 93-0550. Rockville, Md, US Department of Health and Human Services, 1993Google Scholar
31. Greist JH, Jefferson JW: Pharmacotherapy for obsessive-compulsive disorder. Br J Psychiatry Suppl 1988; 35:64-70Google Scholar
32. Davidson JRT: The long-term treatment of panic disorder. J Clin Psychiatry 1998; 59(suppl 8):17-21Google Scholar
33. Davidson JRT: Pharmacotherapy of social anxiety disorder. J Clin Psychiatry 1998; 59(suppl 17):47-51Google Scholar
34. Londborg PD, Hegel MT, Goldstein S, Goldstein D, Himmelhoch JM, Maddock R, Patterson W, Rausch J, Farfel GM: Sertraline treatment of posttraumatic stress disorder: results of a 24-week open-label extension study. J Clin Psychiatry 2001; 62:325-331Crossref, Medline, Google Scholar
35. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218-222Google Scholar
36. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, Keane TM: A clinician rating scale for assessing current and lifetime PTSD: the CAPS-1. Behav Ther 1990; 13:187-188Google Scholar
37. Weathers FW, Litz BT: Psychometric properties of the Clinician-Administered PTSD Scale, CAPS-1. PTSD Res Q 1994; 5:2-6Google Scholar
38. Horowitz MJ, Wilner N, Alvarez W: Impact of Event Scale: a measure of subjective stress. Psychosom Med 1979; 41:209-218Crossref, Medline, Google Scholar
39. Zilberg N, Weiss DS, Horowitz MJ: Impact of Event Scale: a cross validation study and some empirical evidence. J Consult Clin Psychol 1982; 50:407-414Crossref, Medline, Google Scholar
40. Davidson JR, Book SW, Colket JT, Tupler LA, Roth S, David D, Hertzberg M, Mellman T, Beckham JC, Smith RD, Davison RM, Katz R, Feldman ME: Assessment of a new self-rating scale for post-traumatic stress disorder. Psychol Med 1997; 27:153-160Crossref, Medline, Google Scholar
41. Zlotnick C, Davidson J, Shea MT, Pearlstein T: Validation of the Davidson Trauma Scale in a sample of survivors of childhood sexual abuse. J Nerv Ment Dis 1996; 184:255-257Crossref, Medline, Google Scholar
42. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
43. Endicott J, Nee J, Harrison W, Blumenthal R: Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 1993; 29:321-326Medline, Google Scholar
44. Viguera AC, Baldessarini RJ, Friedberg J: Discontinuing antidepressant treatment in major depression. Harv Rev Psychiatry 1998; 5:293-306Crossref, Medline, Google Scholar
45. Fava M, Judge R, Hoog SL, Nilsson ME, Koke SC: Fluoxetine versus sertraline and paroxetine in major depressive disorder: changes in weight with long-term treatment. J Clin Psychiatry 2000; 61:863-867Crossref, Medline, Google Scholar



