Clozapine, Diabetes Mellitus, Weight Gain, and Lipid Abnormalities: A Five-Year Naturalistic Study
Abstract
OBJECTIVE: The goal of this 5-year naturalistic study of patients treated with clozapine was to examine the incidence of treatment-emergent diabetes mellitus in relation to other factors, including weight gain, lipid abnormalities, age, clozapine dose, and treatment with valproate.METHOD: Data on age, gender, race, diagnosis, family history of diabetes, and age at clozapine initiation were collected from medical records of 82 outpatients with schizophrenia or schizoaffective disorder. Clozapine dose, data on use of valproate, and laboratory test results were recorded at 6-month intervals.RESULTS: The mean age at the time of clozapine initiation of the 82 patients was 36.4 years; 26.8% of the patients were women, and 91.5% were Caucasian. The mean baseline weight was 175.5 lb, and the mean body mass index was 26.9 kg/m2. Thirty patients (36.6%) were diagnosed with diabetes during the 5-year follow-up. Weight gain, use of valproate, and total daily dose of clozapine were not significant risk factors for developing diabetes mellitus. Patients experienced significant weight gain that continued until approximately month 46 from initiation of clozapine. There was a nonsignificant increase in total serum cholesterol and a significant increase in serum triglycerides level.CONCLUSIONS: The results support the hypotheses that patients treated with clozapine experience significant weight gain and lipid abnormalities and appear to be at increased risk for developing diabetes.
Clozapine remains the most effective agent for the treatment of refractory schizophrenia, producing response in 30% of patients with treatment-resistant schizophrenia in a 6-week trial (1) and up to 60% at 6 months (2). Although clozapine produces fewer extrapyramidal side effects than conventional neuroleptics, clozapine is not without side effects, and they include agranulocytosis, seizures, sedation, weight gain, constipation, and hypersalivation (3). A program of weekly blood monitoring coordinated by the Clozapine National Registry is credited with reducing the incidence of agranulocytosis from approximately 1%–2% to 0.38% over a 5-year period in the United States (4, 5).
Clozapine is reported to reduce violence, aggression, and suicide in schizophrenia (6, 7). Treatment with clozapine has been reported to reduce the lifetime risk of suicide in patients with schizophrenia from 10%–15% to levels that approach the lifetime risk in general population (8, 9). Walker and colleagues (9) found that mortality from all causes was decreased by clozapine, except for respiratory diseases, pulmonary embolism, and cardiac conduction disorders. Overall, it appears that clozapine reduces a number of factors associated with a high rate of morbidity and mortality in patients with schizophrenia.
However, clozapine has recently been linked to hypertriglyceridemia (10), obesity (11–14), and diabetes (15–19), all risk factors for cardiovascular diseases (20). Several studies have reported substantial weight gain with clozapine (11–14), and weight gain was significantly correlated with clinical response to clozapine in two studies (13, 21). Obesity is associated with an increased risk for hypertension, dyslipidemia, insulin resistance, type 2 (non-insulin-dependent) diabetes mellitus, cardiovascular disease, respiratory dysfunction, and gallstones (22).
Recent case reports have linked clozapine to diabetic ketoacidosis (15–17, 23) and hyperglycemia (24). Popli and colleagues (18) described four cases in which clozapine was associated with exacerbation of diabetes mellitus or new-onset diabetes mellitus not attributable to weight gain. Wirshing and colleagues (19) described four cases of clozapine-associated diabetes and two cases of olanzapine-associated diabetes. Finally, Hagg and colleagues (25) recently reported that 12% of patients treated with clozapine developed type 2 diabetes mellitus and 10% developed impaired glucose tolerance, compared to 6% and 3%, respectively, of patients treated with conventional depot neuroleptics. Although the increased rates of diabetes and impaired glucose tolerance with clozapine did not achieve statistical significance in that study, patients in the conventional neuroleptic group were older, which may have influenced the results. For example, Mukherjee and colleagues (26) found a 15.8% overall prevalence of diabetes in 95 schizophrenia patients. Diabetes occurred in none of the patients younger than 50 years old, in 12.9% of patients age 50–59 years, in 18.9% of patients age 60–69, and in 16.7% of patients age 70–74. Diabetes was significantly more common in neuroleptic-free patients than in patients treated with neuroleptics, after controlling for age, sex, and cumulative duration of neuroleptic treatment. These results exceeded expected rates of type 2 diabetes in the general population, which are 1.3% in persons age 18–44 years, 6.2% in persons age 45–64, and 10.4% in persons age 65 and older (27). Several additional studies have also found increased rates of diabetes or hyperglycemia in schizophrenia but no association with conventional antipsychotic agents (28–30). It is not clear if the increased rates are secondary to increases in insulin-dependent or non-insulin-dependent diabetes mellitus.
Medications have been implicated as potentially impairing glucose metabolism include centrally acting α blockers, β blockers, corticosteroids, cyclosporine, phenytoin, phenothiazines, thiazide diuretics, and oral contraceptives containing norgestrel (28, 31–36). Valproate has been found to induce a metabolic syndrome characterized by centripetal obesity, hyperinsulinemia, lipid abnormalities, polycystic ovaries, and hyperandrogenism in women with epilepsy (37, 38).
The goal of this 5-year naturalistic study was to examine, in patients started on clozapine, the incidence of treatment-emergent impaired glucose tolerance and diabetes mellitus in relation to other factors, including weight gain, serum triglycerides and cholesterol levels, age, and treatment with valproate.
Method
This study was conducted in the outpatient clinic of an urban mental health center. The clinic’s clozapine program began in 1991 after clozapine was introduced to the U.S. market, and by 1993 the program had expanded to care for more than 100 patients. The clinic offers a comprehensive one-stop program in which patients have their blood sampled, attend a psychoeducational group meeting, meet with their psychiatrist or clinical nurse specialist, and receive clozapine from a pharmacist before leaving the clinic. Patients’ vital signs and weight are measured weekly for 4 weeks after initiation of clozapine and then monthly for as long as they are treated with the medication. Screening laboratory tests, performed at baseline and every 6 months, include fasting blood glucose level, electrolytes levels, renal function test, liver function tests, and cholesterol and triglycerides levels. Most patients begin taking clozapine as outpatients.
Data for this study were collected from the medical records and included age, gender, race, diagnosis, family history of diabetes, and age at clozapine initiation. Data on vital signs at 1-month intervals and data on clozapine dose, use of valproate, and laboratory test results at 6-month intervals were recorded from clinical charts. When data were not available, additional information was sought in relevant inpatient records and general medical records.
The diagnosis of diabetes was based on the American Diabetes Association criterion of a fasting blood glucose level equal to or greater than 140 mg/dl on two occasions (39, 40). Patients who experienced one fasting blood glucose equal to or greater than 140 mg/dl were given a nonconfirmed diagnosis of diabetes. Treating psychiatrists were expected to intervene when a fasting blood glucose equal to or greater than 140 mg/dl was reported. Although patients were instructed to fast before the morning blood sampling, it is possible that some of the values were random blood glucose. The data were analyzed by combining definitive cases of diabetes with nonconfirmed cases.
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus in 1997 modified the diagnostic criteria for the diagnosis of diabetes as follows: symptoms of diabetes plus a casual plasma glucose concentration equal to or greater than 200 mg/dl; or a fasting blood glucose level equal to or greater than 126 mg/dl or a 2-hour blood glucose level equal to or greater than 200 mg/dl during an oral glucose tolerance test (41). For epidemiological studies, estimates of diabetes should be based on a fasting blood glucose equal to or greater than 126 mg/dl. Therefore, the data were also analyzed by using this criterion.
This study examines the course of diabetes and weight gain associated with clozapine treatment at 6-month intervals over a 5-year period. Time to development of diabetes or nonconfirmed diabetes, defined as the first follow-up visit at which fasting blood glucose was greater than or equal to 140 mg/dl, was analyzed by means of survival methods. We also present data on patients who were diagnosed and treated for diabetes by their primary care physicians during the 6-month intervals between our fasting blood glucose determinations. Changes in weight, serum cholesterol, and serum triglycerides were treated as continuous variables and analyzed in all patients by means of longitudinal methods.
Proportional hazards regression was used to test the association between the covariates and the development of diabetes. Age and gender were analyzed as baseline covariates, and body mass index, cholesterol level, clozapine total daily dose, triglycerides level, and weight were analyzed as time-dependent covariates. In addition, change from baseline in body mass index, cholesterol and triglycerides levels, and weight were analyzed as time-dependent covariates. Each covariate was analyzed separately by using time as a stratum and handling times with the discrete option in the SAS phreg procedure. This procedure replaces the proportional hazards model with the discrete logistic model so that odds ratios rather than hazard ratios are computed. The Kaplan-Meier estimate of time to development of diabetes was computed. Changes from baseline in weight and cholesterol and triglycerides levels were analyzed by using a mixed-effects model. This model has fixed linear and quadratic terms for time and random intercept and linear and quadratic terms for time for each patient. The fixed effects estimate the mean parabolic trajectory of the change from baseline, and the random effects allow a separate trajectory for each patient.
To test for an association of time-dependent covariates (weight gain, triglycerides level, cholesterol level) with changes from baseline, the mixed-effects model was augmented with a fixed and a random covariate effect. This model tests for a dependency of change from baseline with the covariate beyond that already explained by passage of time. To test for an association between treatment for diabetes and change from baseline, the mixed-effects model was augmented with fixed treatment-by-time and treatment-by-time squared terms. These terms allow a different mean parabolic trajectory in each treatment group. All p values are two-tailed at the significance level of 0.05.
Baseline and follow-up descriptive statistics were tabulated. The incidence of abnormal fasting blood glucose level at each time point was tabulated.
Results
Demographic information and baseline measures are shown in Table 1. Data were obtained for 101 patients who had been treated with clozapine for at least 1 year. Nineteen patients were eliminated from the glucose analysis because of a known history of diabetes mellitus before initiation of clozapine (N=5) or a baseline fasting blood glucose greater than or equal to 140 ng/ml (N=6) or because data on baseline fasting blood glucose or weight were not available (N=8). Of the five patients with a known history of diabetes, two were treated with insulin and three with an oral hypoglycemic agent. Both of the insulin-treated patients required an almost twofold increase in their insulin requirements after initiation of clozapine. Two of the three patients treated with oral agents went on to require insulin after clozapine initiation.
Glucose Analysis
Fasting blood glucose equal to or greater than 140 mg/dl
During the 60-month study, 43 of the 82 patients (52.4%) experienced at least one episode of elevated fasting blood glucose (fasting blood glucose value greater than or equal to 140 mg/dl). Twenty-four patients (29.3%) had one elevated fasting blood glucose value, and 19 (23.2%) had two or more abnormal values. After initiation of clozapine, 25 of the 82 patients (30.5%) were diagnosed with adult-onset type 2 diabetes mellitus by primary care physicians. Seven patients were treated with dietary interventions to manage the diabetes. One of these patients had no abnormal fasting blood glucose values according to our data, but was diagnosed and treated by his primary care physician. Of the seven patients initially treated with dietary interventions, four subsequently required an oral agent.
Fourteen patients were treated with an oral agent. Four patients required treatment with insulin. One of these four patients had only one abnormal fasting blood glucose value, and his condition appeared to be well controlled with insulin treatment. The remaining three had numerous abnormal values and required frequent clinical interventions to manage their diabetes. One patient, a 19-year-old Hispanic man, experienced two episodes of diabetic ketoacidosis within 6 months after initiation of clozapine and required hospitalization in an intensive care unit. His condition may have been unrelated to clozapine and may represent a case of type 1 diabetes mellitus. After this patient’s first episode of diabetic ketoacidosis, his clozapine dose was lowered from 400 mg/day to 200 mg/day and a conventional neuroleptic was added. One month later, the clozapine dose was increased to 300 mg/day because of psychiatric decompensation. Within 4 weeks of the increase, he experienced the second episode of diabetic ketoacidosis. Subsequently, the patient has remained stable on 200 mg/day clozapine, haloperidol decanoate, 100 mg i.m. every 4 weeks, and insulin.
All four patients requiring treatment with insulin gained more than 40% of their body weight (the patient with diabetic ketoacidosis gained more than 100 lb) and experienced abdominal obesity, elevated serum triglycerides, and hypertension.
Surprisingly, 15 patients with one abnormal fasting blood glucose value and five patients with two or more abnormal values had received no treatment or attention related to these findings before data for this study were collected. The five patients with two or more abnormal values were subsequently referred, diagnosed, and treated for diabetes (two were treated with dietary intervention and three with an oral agent). Overall, 30 of 82 patients were diagnosed with diabetes and treated by their primary care physicians (confirmed cases) during the 60-month period. Figure 1 shows the cumulative percentage of patients diagnosed with diabetes mellitus at 6-month intervals during the 60-month period.
Although the small number of non-Caucasian patients precluded an analysis based on race, two of three African American patients and two of three Hispanic patients had abnormal fasting blood glucose values and required treatment for diabetes. One of the two African American patients and one of the two Hispanic patients were treated with insulin, and the others were treated with oral agents.
The proportional hazards regression analysis indicated that age was significantly correlated with the development of diabetes or nonconfirmed diabetes; the odds ratio was 1.07 per year of age (proportional hazards regression, χ2=7.77, df=1, p<0.01). For example, a patient receiving clozapine who was 50 years old has a 3.52 greater chance of developing diabetes than a patient receiving clozapine who is 30 years old (odds ration, where n=the unit (years) difference; in this example, 1.06520=3.52).
Sex was not a significant risk factor for the development of diabetes or nonconfirmed diabetes in this population. Neither were weight, change in weight, body mass index, or change in body mass index associated with the development of diabetes or nonconfirmed diabetes. Overall total serum cholesterol was not associated with development of diabetes or nonconfirmed diabetes. The change in cholesterol over time (1-month units) was nonsignificantly associated with diabetes or nonconfirmed diabetes; the odds ratio was 1.01 per mg/dl cholesterol (proportional hazards regression; χ2=3.73, df=1, p=0.054). For example, an increase in cholesterol of 40 mg/dl increased the risk of developing diabetes to 1.38 (1.00840). Overall serum triglycerides level was nonsignificantly associated with development of diabetes or nonconfirmed diabetes (proportional hazards regression, χ2=2.81, df=1, p=0.094; odds ratio=1.00 per mg/dl), and change in serum triglycerides was also nonsignificantly associated with development of diabetes or nonconfirmed diabetes (proportional hazards regression, χ2=3.76, df=1, p=0.053; odds ratio=1.00 per mg/dl). For example, a patient whose level of serum triglycerides increased by 60 mg/dl had a 1.13 chance of developing diabetes (1.00160) relative to a patient who did not have an increase in serum triglycerides.
There was no association between the daily dose of clozapine (mg/day) or the use of valproate and the development of diabetes or nonconfirmed diabetes. Although family history is a strong predictor in the general population, only two of the 82 patients reported a family history of diabetes.
Fasting blood glucose equal to or greater than 126 mg/dl
During the 60-month study, 55 of 82 patients (67.1%) experienced at least one episode of elevated fasting blood glucose (fasting blood glucose equal to or greater than 126 mg/dl). Twenty-seven (32.9%) had one elevated fasting blood glucose value, and 28 (34.1%) had two or more elevated fasting blood glucose values. Age, sex, weight, change in weight, body mass index, change in body mass index, cholesterol, change in cholesterol, clozapine daily dose, or the use of valproate were not associated with the development of diabetes or nonconfirmed diabetes in this group. Development of diabetes or nonconfirmed diabetes was significantly associated with serum triglycerides level (proportional hazards regression, χ2=4.96, df=1, p=0.03; odds ratio=1.00 per mg/dl) and nonsignificantly associated with change in serum triglycerides level (proportional hazards regression, χ2=3.28, df=1, p=0.07; odds ratio=1.00 per mg/dl).
Weight
The mixed-effects model indicated that weight significantly increased over time among the 82 study patients; the linear coefficient was 1.16 lb/month (SE=0.18) (mixed-effects model, t=6.62, df=80, p=0.0001), and the quadratic coefficient was –0.01 lb/month2 (SE=0.00) (mixed-effects model, t=–4.53, df=79, p=0.0001) (Figure 2). Weight gain also significantly correlated with change in total serum cholesterol (linear coefficient=0.09 lb/mg/dl, SE=0.04; mixed effects model, t=2.41, df=53, p=0.02) and with change in serum triglycerides (linear coefficient=0.02 lb/mg/dl, SE=0.01; mixed-effects model, t=2.86, df=49, p=0.006), when the analysis controlled for time and time squared. Clozapine daily dose (mg/day) did not correlate with weight change nor did any treatment for diabetes. To examine whether weight gain leveled off over time, we analyzed the data from 12 months to 60 months after initiation of clozapine. There was a significant linear coefficient of 0.34 lb/month (SE=0.08) in weight gain from 12 to 60 months (mixed-effects model, t=4.07, df=60, p=0.0001). Patients continued to gain weight until approximately month 46, when weight gain appeared to level off.
Other Variables
There was a nonsignificant increase in total serum cholesterol over the 60-month study period (linear coefficient=0.32 mg/dl per month, SE=0.12; mixed-effects model, t=1.61, df=67, p=0.11). There was a significant increase in serum triglycerides level (linear coefficient=2.75 mg/dl per month, SE=1.28) (mixed-effects model, t=2.14, df=61, p=0.04), which was associated with change in total serum cholesterol (linear coefficient=1.64 mg/dl per mg/dl of total serum cholesterol, SE=0.36) (mixed-effects model, t=4.61, df=60, p=0.0001). The change in serum triglycerides also showed a nonsignificant association with daily clozapine dose (linear coefficient=0.11 mg/dl per mg of clozapine, SE=0.05) (mixed-effects model, t=1.98, df=60, p=0.054). The change in serum triglycerides was not affected by treatment for diabetes.
Discussion
The results of this 5-year naturalistic study confirm our clinical observation and reports in the literature of relatively high rates of new-onset diabetes mellitus during treatment with clozapine. The rate of new-onset diabetes was constant over the 5-year follow-up period and totaled 52% at the end of 5 years, as defined by a single fasting blood glucose equal to or greater than 140 mg/dl, or 67%, as defined by a fasting blood glucose equal to or greater than 126 mg/dl. Perhaps the most compelling finding is that 25 of 82 patients who began taking clozapine (30.5%) were subsequently diagnosed and treated for diabetes by their primary care physicians and an additional five patients were diagnosed after completion of data collection for this study. All told, 30 of 82 patients (36.6%) were diagnosed with diabetes.
In the absence of a comparison group in this study, we must rely on previous studies to estimate the degree to which clozapine elevates rates of diabetes above baseline in patients with schizophrenia. Although some work indicates that patients with schizophrenia have an increased risk of diabetes, it appears that diabetes is uncommon in schizophrenia patients younger than 50 years of age (25). The mean age of the patients in our study was 36.4 years (SD=7.8), and only four (4.9%) were older than age 50. However, schizophrenia may be associated with an increased prevalence of diabetes that is not entirely explained by pharmacological treatment (26). This area requires further clarification with prospective studies.
One difficulty with our method is that we cannot be certain that all glucose determinations were fasting values. This limitation may have resulted in an overestimation of the incidence of diabetes or nonconfirmed diabetes. On the other hand, several patients were diagnosed with diabetes and treated during the 6-month periods between the fasting blood glucose measurements done for this study; after initiation of treatment some of these patients did not have abnormal blood glucose values. A reasonable estimate of the true incidence of new cases of diabetes during the 60-month study period is 36.6%, which is based on the total number of cases diagnosed during the 60-month period (N=25) plus additional cases diagnosed by primary care physicians after data collection (N=5). The rate of onset of diabetes was similar during each of the 6-month intervals throughout the 5-year study period.
The 1997 changes in criteria for the diagnosis of diabetes by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus were intended to more accurately identify patients with diabetes (41). In general, in the United States, diabetes is underdiagnosed. The use of the criterion of a fasting blood glucose value equal to or greater than 126 mg/dl likely resulted in an overestimation of diabetes or nonconfirmed diabetes in this study.
It is surprising that weight gain was not a significant risk factor for developing diabetes. We observed patients who did not gain weight while taking clozapine, but who did develop diabetes, suggesting that mechanisms other than obesity may be involved in non-insulin-dependent diabetes associated with clozapine treatment. However, weight gain was characteristic of patients who developed insulin-requiring diabetes.
Weight gain with clozapine has been well documented (11–14, 21). Patients in our study gained the greatest amount of weight during the first 12 months taking clozapine, and, in contrast to previous reports, they continued to gain weight until approximately month 46. This weight gain occurred despite active weight loss programs involving diet and exercise.
The roles of two important potential risk factors, race and genetics, could not be adequately examined in this study. The majority of published case reports of clozapine-associated diabetes have involved African Americans, who appear to be at a higher risk for diabetes compared to the general population (15–18, 24).
Another potential risk factor is the lack of physical activity. Patients are frequently less active because of the sedating quality of clozapine. Exercise may be a protective factor for type 2 (non-insulin-dependent) diabetes mellitus and glucose intolerance (42). Our patients generally had a diet high in fat and carbohydrate with minimum exercise. During the 5-year period, a number of initiatives to improve dietary habits and exercise were minimally successful and short-lived.
The role of dietary factors also merits further study. Increased dietary intake of saturated fat has been observed in subjects who subsequently develop type 2 (non-insulin-dependent) diabetes mellitus (43, 44). The significant increase in serum triglycerides in our study is consistent with the finding of Ghaeli and Dufresne (10) that subjects taking clozapine had a higher serum triglycerides level than subjects taking conventional antipsychotic agents. Plasma triglycerides may be an independent risk factor for coronary atherosclerosis (45). Many patients in our study received treatment with lipid-lowering agents, which may have affected the results.
The mechanism by which clozapine produces diabetes is not known but could involve suppression of insulin release, insulin resistance, or impairment of glucose utilization. Wirshing and colleagues (19) suggested that clozapine’s interaction with specific serotonin receptors may result in these metabolic abnormalities.
The data suggest that, if clozapine affects glucose metabolism, the elevated risk of developing diabetes continues as long as the treatment. Further controlled studies are required to determine the magnitude and mechanism of clozapine’s impairment of glucose metabolism. We recommend that patients who take clozapine be screened every 6 months for diabetes and lipid abnormalities with a fasting blood glucose determination. When such an assessment is difficult because of the timing of the blood sampling or poor patient compliance with fasting, random blood glucose measurement should be used. Any abnormal result should be followed up with more accurate tests, such as a fasting blood glucose test, measurement of glycohemoglobin, or an oral glucose tolerance test. We were struck by how often the treating psychiatrist ignored abnormal blood glucose results, and we suspect this pattern may occur in other mental health centers. We encourage psychiatrists to play an active role in the medical care of their patients with chronic mental illness, which includes monitoring and responding to regular screening laboratory tests as well as recording weight and blood pressure monthly. Appropriate interventions in addition to health education and smoking cessation programs may further reduce medical morbidity and mortality in patients treated with clozapine.
 |
Received April 12, 1999; revisions received Aug. 19 and Oct. 14, 1999; accepted Dec. 14, 1999. From the Psychotic Disorders Program, Diabetes Clinical Research Center, and Biostatistics Program, Massachusetts General Hospital, Boston; and Northeastern University, Boston. Address reprint requests to Dr. Henderson, Freedom Trail Clinic, Harvard Medical School, 25 Staniford Street, Boston, MA 02114; [email protected] (e-mail) The authors thank Christina P. Borba and Christine E. Connolly.
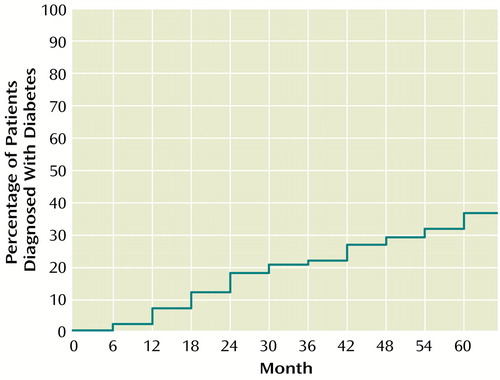
Figure 1. Cumulative Percentage of Patients Diagnosed With Diabetes Mellitus (N=30) Over a 60-Month Period Among 82 Patients Receiving Clozapine
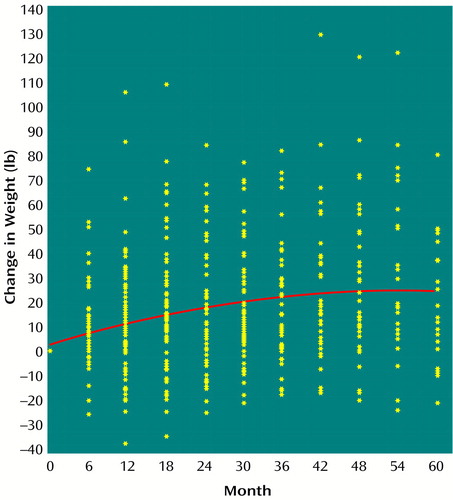
Figure 2. Change in Weight From Baseline During a 60-Month Study in 82 Patients Receiving Clozapinea
aSolid curve shows the quadratic fit to all available data.
1. Kane J, Honigfeld G, Singer J, Meltzer H: Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
2. Meltzer HY, Burnett S, Bastani B, Ramirez LF: Effect of six months of clozapine treatment on the quality of life of chronic schizophrenic patients. Hosp Community Psychiatry 1990; 41:892–897Abstract, Google Scholar
3. Kane JM: Clinical efficacy of clozapine in treatment-refractory schizophrenia: an overview. Br J Psychiatry Suppl 1992; 17:41–45Medline, Google Scholar
4. Honigfeld G: The Clozapine National Registry system: forty years of risk management. J Clin Psychiatry Monograph 1996; 14:29–32Google Scholar
5. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J: Reducing clozapine-related morbidity and mortality:5 years of experience with the Clozapine National Registry. J Clin Psychiatry 1998; 59(suppl 3):3–7Google Scholar
6. Glazer WM, Dickson RA: Clozapine reduces violence and persistent aggression in schizophrenia. J Clin Psychiatry 1998; 59(suppl 3):8–14Google Scholar
7. Meltzer HY: Suicide in schizophrenia: risk factors and clozapine treatment. J Clin Psychiatry 1998; 59(suppl 3):15–20Google Scholar
8. Harris EC, Barraclough B: Excess mortality of mental disorder. Br J Psychiatry 1998; 173:11–53Crossref, Medline, Google Scholar
9. Walker AM, Lanza LL, Arellano F, Rothman KJ: Mortality in current and former users of clozapine. Epidemiology 1997; 8:671–677Crossref, Medline, Google Scholar
10. Ghaeli P, Dufresne RL: Serum triglyceride levels in patients treated with clozapine. Am J Health Syst Pharm 1996; 53:2079–2081Google Scholar
11. Umbricht DSG, Pollack S, Kane JM: Clozapine and weight gain. J Clin Psychiatry 1994; 55(Sept suppl B):157–160Google Scholar
12. Bustillo JR, Buchanan RW, Irish D, Breier A: Differential effect of clozapine on weight: a controlled study. Am J Psychiatry 1996; 153:817–819Link, Google Scholar
13. Lamberti JS, Bellnier T, Schwarzkopf SB: Weight gain among schizophrenic patients treated with clozapine. Am J Psychiatry 1992; 149:689–690Link, Google Scholar
14. Cohen S, Chiles J, MacNaughton A: Weight gain associated with clozapine. Am J Psychiatry 1990; 147:503–504Link, Google Scholar
15. Koval MS, Rames LJ, Christie S: Diabetic ketoacidosis associated with clozapine treatment (letter). Am J Psychiatry 1994; 151:1520–1521Google Scholar
16. Kostakoglu AE, Yazici KM, Erbas T, Guvener N: Ketoacidosis as a side-effect of clozapine: a case report. Acta Psychiatr Scand 1996; 93:217–218Crossref, Medline, Google Scholar
17. Peterson GA, Byrd SL: Diabetic ketoacidosis from clozapine and lithium cotreatment (letter). Am J Psychiatry 1996; 153:737–738Medline, Google Scholar
18. Popli AP, Konicki PE, Jurjus GJ, Fuller MA, Jaskiw GE: Clozapine and associated diabetes mellitus. J Clin Psychiatry 1997; 58:108–111Crossref, Medline, Google Scholar
19. Wirshing DA, Spellberg BJ, Erhart SM, Marder SR, Wirshing WC: Novel antipsychotics and new onset diabetes. Biol Psychiatry 1998; 44:778–783Crossref, Medline, Google Scholar
20. Amdisen A: Drug-produced obesity: experiences with chlorpromazine, perphenazine, and clopenthixol. Dan Med Bull 1964; 11:182–189Medline, Google Scholar
21. Leadbetter R, Shutty M, Pavalonis D, Viewig V, Higgins P, Downs M: Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry 1992; 149:68–72Link, Google Scholar
22. Pi-Sunyer FX: Medical hazards of obesity. Ann Intern Med 1993; 119(7, part 2):655–660Google Scholar
23. Smith H, Kenney-Herbert J, Knowles L: Clozapine-induced diabetic ketoacidosis. Aust NZ J Psychiatry 1999; 33:121–122Crossref, Medline, Google Scholar
24. Kamran A, Doraiswamy PM, Jane JL, Hammett EB, Dunn L: Severe hyperglycemia associated with high doses of clozapine (letter). Am J Psychiatry 1994; 151:1395Medline, Google Scholar
25. Hagg S, Joelsson L, Mjorndal T, Spigset O, Oja G, Dahlqvist R: Prevalence of diabetes and impaired glucose tolerance in patients treated with clozapine compared with patients treated with conventional depot neuroleptic medications. J Clin Psychiatry 1998; 59:294–299Crossref, Medline, Google Scholar
26. Mukherjee S, Decina P, Bocola V, Saracini F, Scapicchio PL: Diabetes mellitus in schizophrenic patients. Compr Psychiatry 1996; 37:68–73Crossref, Medline, Google Scholar
27. Kenny SJ, Aubert RE, Geiss LS: Prevalence and incidence of non-insulin-dependent diabetes, in Diabetes in America, 2nd ed: NIH Publication 95-1468. Bethesda, Md, National Institute of Diabetes and Digestive and Kidney Diseases, 1995, pp 47–68Google Scholar
28. Keskiner A, el-Toumi A, Bousquet T: Psychotropic drugs, diabetes and chronic mental patients. Psychosomatics 1973; 14:176–181Crossref, Medline, Google Scholar
29. Mukherjee S, Roth SD, Sandyk R, Shnur DB: Persistent tardive dyskinesia and neuroleptic effects on glucose tolerance. Psychiatry Res 1989; 29:17–27Crossref, Medline, Google Scholar
30. Schwartz L, Munoz R: Blood sugar levels in patients treated with chlorpromazine. Am J Psychiatry 1968; 125:253–255Link, Google Scholar
31. Pandit MK, Burke J, Gustafson AB, Minocha A, Peiris AN: Drug-induced disorders of glucose tolerance. Ann Intern Med 1993; 118:529–539Crossref, Medline, Google Scholar
32. Bowes WA, Katta LR, Droegemeuller W, Bright TG: Triphasic randomized clinical trial: comparison of effects on carbohydrate metabolism. Am J Obstet Gynecol 1989; 161:1402–1407Google Scholar
33. Wynn V: Effects of duration of low-dose oral contraceptive administration on carbohydrate metabolism. Am J Obstet Gynecol 1982; 162:739–746Google Scholar
34. Thonnard-Neuman E: Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968; 124:978–982Link, Google Scholar
35. Proakis AG, Mennear JH, Miya TS, Borowitz JL: Phenothiazine-induced hyperglycemia: relation to CNS and adrenal effects. Proc Soc Exp Biol Med 1971; 137:1385–1388Google Scholar
36. Tollefson G, Lesar T: Nonketotic hyperglycemia associated with loxapine and amoxapine: case report. J Clin Psychiatry 1983; 44:347–348Medline, Google Scholar
37. Isojarvi JIT, Rattya J, Myllyla VV, Knip M, Koivunen R, Pakarinen AJ, Tekay A, Tapanainen JS: Valproate, lamotrigine, and insulin-mediated risks in women with epilepsy. Ann Neurol 1998; 43:446–451Crossref, Medline, Google Scholar
38. Isojarvi JI, Laatikainen TJ, Pakarinen AJ, Juntunen KT, Myllyla VV: Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med 1993; 329:1383–1388Google Scholar
39. National Diabetes Data Group: Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979; 28:1039–1057Google Scholar
40. World Health Organization: Diabetes Mellitus: Report of a WHO Study Group: Technical Report 727. Geneva, WHO, 1985Google Scholar
41. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20:1183–1197Google Scholar
42. Zimmet PZ: Kelly West Lecture 1991: Challenges in diabetes epidemiology—from West to the rest. Diabetes Care 1992; 15:232–252Crossref, Medline, Google Scholar
43. Marshall JA, Shetterly S, Baxter J, Murmman RF: Dietary fat as a risk factor for conversion from impaired glucose tolerance (IGT) to non-insulin dependent diabetes mellitus (NIDDM): the San Luis Valley Diabetes Study (abstract). Diabetes 1990; 40(suppl 1):1500AGoogle Scholar
44. Feskens EJM, Krombout D: Habitual dietary intake and glucose intolerance in euglycaemic men: the Zutphen Study. Int J Epidemiol 1990; 19:953–959Crossref, Medline, Google Scholar
45. Drexel H, Amann FW, Beran J, Rentsch K, Candinas R, Muntwyler J, Luethy A, Gasser T, Follath F: Plasma triglycerides and three lipoprotein cholesterol fractions are independent predictors of the extent of coronary atherosclerosis. Circulation 1994; 90:2230–2235Google Scholar



