Valproate Prescription Prevalence Among Women of Childbearing Age
Abstract
Objective:
Valproate is associated with polycystic ovary syndrome as well as congenital malformations and developmental delays of infants who were prenatally exposed. The frequency of valproate use for mental health conditions among women of childbearing age was determined.
Methods:
Using New York State Medicaid claims for persons with psychiatric disorders, 40,526 individuals with active prescriptions for mood stabilizers (non-antipsychotic) on May 1, 2009, were identified. Chi square tests were used to compare valproate use among women of childbearing age with similarly aged men and older women.
Results:
Valproate was the most commonly prescribed agent for young women (23.4%). Men were more likely than women, and older women more likely than younger women, to take valproate.
Conclusions:
Over 20% of childbearing-aged women receiving mood stabilizers were treated with valproate, although increasing data on the reproductive toxicity of this agent compel consideration of other non-antipsychotic mood stabilizers as first-line choices. (Psychiatric Services 62:218–220, 2011)
Valproate is widely used to treat bipolar disorder. Two lines of research related to the impact of valproate on reproductive health are relevant for treating women with mental health conditions. First, valproate increases risk for the development of polycystic ovary syndrome, an endocrine disorder characterized by hyperandrogenism, obesity, reduced fertility, insulin resistance, lipid abnormalities, and cardiovascular disease. Joffe and colleagues (1) reported that hyperandrogenism with oligoamenorrhea developed with nine of 86 (11%) women treated with valproate and with two of 144 (1%) women who took another anticonvulsant or lithium (relative risk=7.5, 95% confidence interval [CI]=1.7–34.1, p=.002).
Second, valproate increases the rates of birth defects and developmental delay of offspring exposed antenatally. Gestational valproate exposure is associated with higher rates of serious adverse outcomes, such as congenital malformations and fetal death, compared with other anticonvulsants (2): lamotrigine 1.0%, carbamazepine 8.2%, phenytoin 10.7%, and valproate 20.3%. Compared with other antiepileptic drugs, valproate is associated with the highest rate of birth defects (10.7%; CI=8.2–13.3) (3). The Food and Drug Administration recently posted (December 3, 2009) a safety bulletin on the risk of neural tube defects after prenatal exposure to valproate (www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm192788.htm).
Valproate also affects infant neurodevelopment. Three-year-old children exposed to valproate in utero had average IQ scores 9 points lower than those exposed to lamotrigine (95% CI=3.1–14.6, p=.009) and 6 points lower than those exposed to carbamazepine (95% CI=.6–12.0, p=.04) (4). Because over half of American women's pregnancies are unplanned and the negative impact on neural tube development occurs 17–30 days after conception, women treated with maintenance valproate have exposure during this critical period before pregnancy recognition. These findings dictate against the use of valproate as a first-line treatment for childbearing-aged women (4,5). We determined the rates of valproate use for women of childbearing age to assess the current prescribing prevalence.
Methods
The New York State Office of Mental Health (OMH) receives Medicaid claims data from the New York State Department of Health for individuals who either have a psychiatric diagnosis or who have generated a Medicaid claim for a mental health service in the current or prior year. The study sample consisted of Medicaid recipients with one or more active non-antipsychotic mood stabilizer prescriptions on May 1, 2009. Individuals with a diagnosis of a seizure disorder (ICD-9 diagnoses codes 345–345.99) in the previous five years were excluded. This work was conducted as part of a quality improvement initiative and was deemed by the OMH institutional review board to not constitute human subjects research.
Demographic information was extracted from Medicaid records (date of birth, gender, and race and ethnicity). We used the World Health Organization's definition of childbearing age (15–49 years) on the focal day (May 1, 2009) to identify the following groups: women of childbearing age, men in the same age range (15–49), and women 50 years and older.
Following a procedure created at the University of Pittsburgh (B. Stein, personal communication, May 21, 2008), we used claims data to assign a diagnosis according to the one most frequent among the ten most recent service claims in the past year. If a tie occurred, the most recent diagnosis was assigned, and if two diagnoses occurred on the same day, we selected the one associated with the higher-priced claim.
Frequency distributions were developed for race, gender, and diagnosis. Rates of use of non-antipsychotic mood stabilizers were examined for the entire sample and for three subgroups: women aged 15–49, men aged 15–49, and women ≥50 years of age. Chi square tests were used to compare rates of valproate use between subgroups.
Results
Of 48,028 individuals with an active prescription for a non-antipsychotic mood stabilizer, 7,502 were not included because of a diagnosis of epilepsy, for a study sample of 40,526. A total of 51.3% were Caucasian (N=20,781); 21.0% (N=8,508) were African American, 12.7% (N=5,150) were Latino, 9.5% (N=3,837) were from other racial-ethnic minority groups, and 5.5% (N=2,250) had missing information. A total of 23,196 (57.2%) were women with a mean age of 38.8±14.8 years. The age categories included 3.4% (N=791) <15 years; 61.4% (N=14,236) 15–49 years, and 35.2% (N=8,169) ≥50 years of age. For men, 10.9% (N=1,883) were <15 years, 62.5% (N=10,823) 15–49 years, and 26.7% (N=4,624) ≥50 years. Among 40,526 individuals taking a non-antipsychotic mood stabilizer, 24.0% (N=9,744) had a bipolar disorder, 22.9% (N=9,298) had major depression or dysthymia, 18.7% (N=7,566) had schizophrenia, 8.5% (N=3,458) had an anxiety disorder, 13.3% (N=5,394) had other diagnoses, and 12.5% (N=5,066) were missing data that indicated psychiatric diagnosis. Individuals in the sample had 44,726 active prescriptions for non-antipsychotic mood stabilizers.
In the entire sample, valproate was the most frequently prescribed non-antipsychotic mood stabilizer (N=13,125, 32.3%), followed by gabapentin (N=10,697, 26.4%), lamotrigine (N=6,757, 16.7%), topiramate (N=5,460, 13.5%), lithium (N=5,293, 13.0%), oxcarbazepine (N=2,080, 5.1%), and carbamazepine (N=1,314, 3.2%). Women of childbearing age were less likely to be prescribed valproate compared with similarly aged men (23.4% versus 44.3%, χ2=1,222.4, df=1, p<.001) or women ≥50 years old (23.4% versus 25.8%, χ2=16.2, df=1, p<.001) (Figure 1). Compared with women ≥50 years, childbearing-aged women were more likely be prescribed lithium (12.4% versus 8.1%, χ2=99.6, df=1, p<.001), although less likely compared with similarly aged men (16.9%, χ2=98.5, df=1, p<.001). For women ≥50 years, gabapentin was the most commonly prescribed non-antipsychotic mood stabilizer and was prescribed significantly more often than for younger women (41.9% versus 22.9%, χ2=892.2, df=1, p<.001).
Discussion
Valproate was the most frequently prescribed non-antipsychotic mood stabilizer for women of childbearing age. Among individuals on a non-antipsychotic mood stabilizer, women of childbearing age had lower rates of receiving valproate than similarly aged men or older women; however, 23.4% of women received this agent. Lithium, carbamazepine, and lamotrigine have less reproductive toxicity than valproate (5–7), and guidelines with risk-benefit decision-making support for treatment of pregnant women with bipolar disorder have been published (7). In the United Kingdom, Ackers and colleagues (8) demonstrated a tenfold increase in lamotrigine prescriptions for adolescent girls with epilepsy from 1993 to 2006, with fewer prescriptions for valproate and carbamazepine because of the greater reproductive toxicity of these agents.
The filling of a prescription by a woman of childbearing age provides an opportunity to provide teratology information to patients and prescribers. Our data suggest that such opportunities are frequent and could be incorporated into quality improvement initiatives to emphasize treatment with evidence-based agents tailored to reproductive capacity.
Even after data for individuals with a seizure disorder were removed from the analysis, patients with a wide variety of psychiatric diagnoses received anticonvulsants, and only a minority (20%) had diagnoses of bipolar disorder. The research base supports valproate, lamotrigine, and carbamazepine, but not gabapentin or other anticonvulsive agents, as being effective treatments for bipolar disorder (9,10). Nevertheless, gabapentin was the second most prescribed anticonvulsant in the New York State Medicaid mental health population and the most common agent prescribed to older women.
Conclusions
More than 20% of childbearing-aged women who had a psychiatric disorder and received a non-antipsychotic mood stabilizer were treated with valproate. The benefit of valproate treatment may outweigh the risks, such as for young women who are nonresponsive to alternative mood stabilizers. However, reducing the rate of first-line valproate use among women of childbearing age creates an opportunity for prevention of reproductive toxicity. Informing patients and providers about the comparative reproductive risks among mood stabilizers as well as about the evidence to support the efficacy of only a subset of anticonvulsants for bipolar disorder is a necessary component of influencing practice patterns (10). Researchers and quality improvement administrators should collaborate to develop interventions to support evidence-based treatments that incorporate reproductive considerations.
1 : Valproate is associated with new-onset oligomenorrhea with hyperandrogenism in women with bipolar disorder. Biological Psychiatry 59:1078–1086, 2006 Crossref, Medline, Google Scholar
2 : In utero antiepileptic drug exposure: fetal death and malformations. Neurology 67:407–412, 2006 Crossref, Medline, Google Scholar
3 : Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Research 81:1–13, 2008 Crossref, Medline, Google Scholar
4 : Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. New England Journal of Medicine 360:1597–1605, 2009 Crossref, Medline, Google Scholar
5 : Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 73:133–141, 2009 Crossref, Medline, Google Scholar
6 : A reevaluation of risk of in utero exposure to lithium. JAMA 271:146–150, 1994 Crossref, Medline, Google Scholar
7 : Management of bipolar disorders during pregnancy and the postpartum period. American Journal of Psychiatry 161:608–620, 2004 Link, Google Scholar
8 : Changing trends in antiepileptic drug prescribing in girls of child-bearing potential. Archives of Disease in Childhood 94:443–447, 2009 Crossref, Medline, Google Scholar
9 : Anticonvulsants in bipolar disorders: current research and practice and future directions. Bipolar Disorders 11(suppl 2):20–33, 2009 Crossref, Medline, Google Scholar
10 : Disseminating findings from a drug class review: using best practices to inform prescription of antiepileptic drugs for bipolar disorder. Journal of Psychiatric Practice 14(suppl 1):44–56, 2008 Crossref, Medline, Google Scholar
Figures and Tables
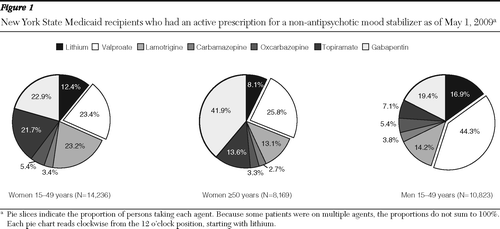
Figure 1 New York State Medicaid recipients who had an active prescription for a non-antipsychotic mood stabilizer as of May 1, 2009



