After the Black Box Warning: Predictors of Psychotropic Treatment Choices for Older Patients With Dementia
Abstract
Objectives:
This study aimed to evaluate factors associated with the selection of pharmacological treatments often given as first-line treatments to elderly patients with neuropsychiatric symptoms associated with dementia. It also evaluated patterns of medication usage over time in the year preceding and three years after the U.S. Food and Drug Administration issued a black box warning for antipsychotic usage.
Methods:
A retrospective cohort consisted of 19,517 Veterans Affairs patients with diagnosed dementia and a new outpatient start with an antipsychotic agent (haloperidol, olanzapine, quetiapine, or risperidone) or valproic acid and its derivatives between May 1, 2004, and September 30, 2008. Patient and facility characteristics were examined for their association with the new starts of these medications.
Results:
Trends in the rate of fills for psychotropic medications varied, with yearly increases in the use of quetiapine, haloperidol, and valproic acid and decreasing use of olanzapine and risperidone. Predictors of haloperidol use included a new start in nonpsychiatric settings, prior benzodiazepine use, and any prior-year hospitalization. Anxiety disorder and major depression were predictive of not receiving haloperidol. Parkinson's disease was predictive of quetiapine use, whereas bipolar disorder was predictive of valproic acid use. Older age was predictive of use of antipsychotics rather than valproic acid. Urban facilities were less likely to use olanzapine, and significant regional variations were seen.
Conclusions:
Important patient and facility characteristics were associated with initiating different psychotropic agents among elderly dementia patients. In addition, the rate of use and the factors predictive of use varied across the study years. (Psychiatric Services 62:1207–1214, 2011)
Although no medication has been approved by the U.S. Food and Drug Administration (FDA) to treat the behavioral symptoms of dementia, antipsychotics have long been used for such symptoms. In 2005, based on a meta-analysis of randomized controlled trial (RCT) data, the FDA issued a warning that second-generation antipsychotic treatment of the behavioral disturbances associated with dementia was associated with increased mortality (1). Subsequent studies confirmed concerns about mortality with second-generation and first-generation antipsychotic medications for elderly patients with dementia as well as their higher risk compared with other psychotropic medications (2–6).
With the exception of one meta-analysis of RCT data, the aforementioned studies were observational (2). Researchers, drug safety experts, and policy makers are increasingly using large clinical observational data to evaluate safety and explore relationships between medication use and adverse outcomes (5,6) because conducting RCTs with sufficient sample sizes to address rare serious adverse event outcomes, such as mortality or cardiovascular events, is difficult. Observational studies have important advantages when studying long-term outcomes: large numbers of patients can be followed for extended periods at reasonable expense, and the adoption of sophisticated information technology by large organized health care systems, such as the Veterans Health Administration (VHA), has created new opportunities for following large patient samples with detailed treatment information for long periods. However, a major limitation in using existing administrative or clinical data sets is treatment selection. In real-world clinic settings, the choice of agent for a given patient is potentially affected by a number of factors, including patient demographic characteristics, comorbid medical and psychiatric issues, and health care utilization profile. To draw valid conclusions from observational studies comparing risks between medications, it is important to understand trends in prescribing and predictors of selecting individual medications that may be associated with outcomes. However, provider treatment choice of medications for behavioral symptoms of dementia has received limited attention.
Our goal was to evaluate factors associated with the use of pharmacological treatments typically given as first-line treatment to elderly outpatients with behavioral symptoms of dementia, as well as to evaluate patterns of medication usage over time. Using national VHA data with comprehensive diagnosis and pharmacy information, we examined which patient and facility characteristics available in the administrative data were predictive of initial medication choice. Medications included were the most commonly used antipsychotic agents (risperidone, olanzapine, quetiapine, and haloperidol) as well as valproic acid and its derivatives (sodium valproate and divalproex). Inclusion of valproic acid and its derivatives, hereinafter called valproic acid, was based on evidence from our earlier work reflecting common use among dementia patients and showing mortality risks similar to those of antipsychotics (7).
Methods
Study population and design
We used data from national VHA registries maintained by the Serious Mental Illness Treatment Resource and Evaluation Center in Ann Arbor, Michigan, for veterans who received a dementia diagnosis (ICD-9 diagnoses 290.0, 290.1x, 290.2x, 290.3, 290.4x, 291.2, 294.10, 294.11, 331.0, 331.1, and 331.82) in a VHA setting. The retrospective cohort included all patients age 65 years or older at the time of their first new outpatient start of antipsychotics or valproic acid between May 1, 2004, and September 30, 2008. A new medication start was determined as having a period with no antipsychotic or anticonvulsant fill in the previous 12 months. Anticonvulsant medications were considered to be used for behavioral symptoms of dementia only if patients did not have a concurrent diagnosis of a seizure disorder, and thus patients with seizure diagnoses during one year prior to and including the time of the anticonvulsant fills were excluded. This study was approved by the institutional review board of the Veterans Affairs Ann Arbor Health System.
Study variables
Patients' gender, age, race (black, white, other, or unknown), ethnicity (Hispanic or non-Hispanic), and marital status were ascertained from national VHA databases. Dementia was further broken down into smaller groups comprising more specific dementia diagnoses: Alzheimer's disease, vascular dementia, Lewy body or Parkinson's dementia (DLBD/PDD), other dementias (including alcoholic dementia and Pick's disease), and a “mixed” category of dementia for patients with more than one dementia subtype. We also calculated days since first dementia diagnosis as a proxy for dementia severity as in our prior work (6). All diagnosis, prior medication use, and utilization data were based on data during the 12 months before the start of new medication.
Comorbid psychiatric diagnoses included posttraumatic stress disorder (PTSD), personality disorders, major depression, other anxiety disorders, bipolar disorder, alcohol abuse or dependence, other substance abuse or dependence, tobacco use disorder, delirium, other depression, schizophrenia or schizoaffective disorder, and other psychotic disorders. Overall medical burden was measured with a modified version of the Charlson Comorbidity Index based on 18 medical comorbidities (excluding dementia) (8). Service utilization data included number of inpatient stays and number of nursing home days. Facility variables where the medication was filled included geographic region, rurality based on metropolitan statistical area designation, academic affiliation, and number of beds. As an indication of whether the psychotropic medication was prescribed by a psychiatrist or another type of physician, we included whether an outpatient psychiatric visit was made within 30 days of the fill. We also included fiscal year at the start of the new medication to control for trends.
Data analyses
Descriptive statistics were calculated as percentages or means and standard deviations. The primary outcome was choice of one of five newly started psychotropic medications. Predictors of the medication choices were examined with multinomial logistic regression models (9). For continuous variables, we checked for functional relationships and categorized the variables appropriately. Using Huber-White sandwich estimators to account for potential correlation within facility, we obtained robust standard errors with clustering by facility (10). Because of significant yearly variation in use, we explored whether factors associated with initial choice of medication remained similar across years by fitting the models separately by fiscal year. Any notable changes across years in the direction or magnitude of the factors associated with the selection of the medication were tested for statistical significance by including interactions of those factors with fiscal years. All analyses were conducted with Stata 11.1 (College Station, Texas).
Results
The study included 19,517 patients with dementia who were 65 or older. Most were male (97.5%) and white (73.2%). Table 1 shows the distribution of the medication types by fiscal year (FY). The number of patients with new medication starts decreased from 4,945 in FY 2005 to 3,872 in FY 2008. Averaged across the years, quetiapine was most widely used, followed by risperidone. The rate of new medication starts varied widely over the years; risperidone and olanzapine fills decreased over time, while quetiapine, haloperidol, and valproic acid fills increased (Figure 1). In particular, olanzapine starts declined by more than half, from 13.8% in FY 2004 to 6.6% in FY 2008, while valproic acid starts doubled from 5.1% to 10.2%.
Table 2 shows significant variation in the selection of medication by dementia subtypes (p<.001). Across medications, the most common dementia subtype was Alzheimer's, affecting 78.6% of patients. Olanzapine and risperidone were more commonly used to treat Alzheimer's, haloperidol and valproic acid were most commonly used to treat vascular dementia, with quetiapine for DLBD/PDD, valproic acid for other dementias, and quetiapine for mixed dementias.
Table 3 shows patient and facility characteristics associated with each medication. Compared with patients using the other medications, significantly fewer patients treated with haloperidol had a psychiatric visit associated with the new start (19.5%). Compared with users of the other medications, a significantly larger percentage of patients starting haloperidol treatment were African American. Haloperidol patients were more likely to have had a benzodiazepine fill in the prior year (24%) and were less likely to have had a prior antidepressant fill (45.4% versus >50% for other antipsychotics). Other anxiety disorders and depression were less prevalent among haloperidol users than users of other medication. On the other hand, more than 50% of haloperidol patients had diagnoses of delirium, and haloperidol starts were associated with a greater number of prior inpatient hospital days and number of comorbid illnesses. Nearly 15% of the quetiapine patients had Parkinson's disease, and a greater percentage of patients taking valproic acid had a bipolar disorder. The median number of days since dementia diagnosis (as a proxy for dementia stage) was highest among patients who filled a prescription for valproic acid followed by those who filled prescriptions for haloperidol.
Table 4 gives the adjusted relative risk ratio (RRR) estimates in relation to risperidone, the most commonly used agent to treat dementia among veterans. Because the study used a large number of patients, we generally emphasize the significant predictors with the estimated RRRs >1.25 or <.8.
Patients' demographic characteristics remained predictive of the choice of initial medication. Compared with the youngest older patients (65–70 years), older patients were less likely to start taking valproic acid and its derivatives compared with risperidone. African-American patients were less likely to fill valproic acid compared with risperidone. Among the dementia subtypes, compared with Alzheimer's-type dementia, vascular dementia patients were 1.23 times more likely to start on valproic acid than on risperidone. Patients with mixed dementias were also more likely to start on valproic acid or quetiapine compared with risperidone, and DLBD/PDD patients were 3.05 times more likely than Alzheimer's patients to start on quetiapine versus risperidone. Compared with risperidone, haloperidol was .47 times less likely to be filled by those with at least one outpatient psychiatric visit in the prior 30 days of the fill, at least 1.64 times more likely to be filled by those with one or more hospitalization days in the prior year, and 1.57 times more likely to be filled by prior benzodiazepine users. Depression diagnosis was less likely among haloperidol patients, and prior antidepressant use was associated more strongly with the use of valproic acid, olanzapine, and quetiapine than with use of risperidone. Bipolar disorder types 1 and 2 were more associated with prescriptions for valproic acid than for risperidone, and Parkinson's disease was more associated with olanzapine than risperidone and most notably 3.67 times more likely to be associated with quetiapine than risperidone. Patients with schizophrenia or schizoaffective diagnoses were more likely to receive a prescription for olanzapine and less likely to receive one for valproic acid compared with risperidone.
Trends seen across years for each medication remained significant. Specifically, compared with risperidone, we found that quetiapine, haloperidol, and valproic acid were all more likely to be filled over the study years, but olanzapine became less likely than risperidone to be filled. Significant variation across facility characteristics was found as well. Compared with patients from the South, Midwestern patients were less likely to fill prescriptions for haloperidol and quetiapine compared with risperidone. Urban facility patients were less likely than rural facility patients to receive olanzapine than risperidone, and facilities with high academic affiliation were more likely than those with limited or low academic affiliation to use olanzapine and quetiapine than risperidone.
Exploratory analyses showed notable changes across the years in the prescribing pattern associated with facility characteristics. Patients from facilities with high academic affiliation were more likely than patients from facilities with limited or low academic affiliation to start on haloperidol in 2004, but they were less likely to start on haloperidol in later years. On the other hand, valproic acid was less likely to be filled in high academically affiliated facility patients in FY 2004, but by FY 2008, no difference was seen.
Discussion
Although we found that the total number of elderly patients with dementia who newly started on one of five agents decreased slightly from FY 2005 (the year of the FDA warning) (1) to FY 2008, the rate of new quetiapine fills increased from 35.4% in FY 2004 to 43.4% in FY 2008 (Figure 1). Interestingly, rates of valproic acid and haloperidol use also increased during the same period.
Race was a significant factor even after we adjusted for baseline variables. The only first-generation antipsychotic examined, haloperidol, was more likely than olazapine, quetiapine, or valproic acid to be prescribed among African-American patients (p=.009, p=.01, and p=.001, respectively, in post hoc tests). Higher use of first-generation antipsychotics among African-American patients compared with Caucasian patients has been previously documented (11,12). Haloperidol was also used less frequently than second-generation antipsychotics among patients with at least one prior psychiatric outpatient visit. Although cultural or ethnic bias has been raised as a potential cause for this disparity, the higher rate of haloperidol starts among African-American patients may also be associated with differences in health care utilization by race in that haloperidol was more likely to be prescribed in primary care settings. African-American patients have been reported to use mental health care at significantly lower rates than Caucasian patients (13,14). Haloperidol was also less likely than risperidone to be filled by patients with prior antidepressant fills or comorbid psychiatric diagnoses (including depression, bipolar disorder types 1 and 2, and other anxiety disorders), whereas it was more likely to be filled by those with delirium, comorbid general medical illnesses, prior inpatient stays, or prior benzodiazepine use. Lower haloperidol use in the Midwest may indicate that geographic factors play a role as well.
Relative increases in haloperidol use over the study period are intriguing, particularly because in earlier work we found a significant decline in first-generation antipsychotic use predating the black box warnings (7). The shift from first- to second-generation antipsychotics with the elderly population during this period has been hypothesized to be due to several factors: efficacy evidence from early clinical trials, perceived safety advantages at that time, and published expert consensus guidelines (15). After the black box warning was issued, our prior work found that the use of first-generation antipsychotics among VHA patients with dementia was less than 2%. Given greater rates of use by those with physical comorbid illnesses, prior inpatient stays, and longer times since dementia diagnoses, increases in haloperidol use in the post-black box warning period may be related to increased recognition and treatment of delirium, a syndrome commonly seen among patients with dementia. Although there is no evidence of greater efficacy over other antipsychotics in treating delirium, haloperidol is widely considered the standard of care (16).
Use of individual second-generation antipsychotics varied with psychiatric and medical diagnoses. Comorbid bipolar disorder, schizophrenia, and schizoaffective disorder were predictive of use of olanzapine over other medications, and comorbid bipolar disorder was predictive of use of valproic acid over other medications. This finding may be related to greater perceived efficacy among patients with serious mental illness, particularly in light of the findings of the Clinical Antipsychotic Trials of Intervention Effectiveness (17). The increased use of quetiapine among patients with Parkinson's disease or DLBD/PDD was expected, in that olanzapine and risperidone are both associated with movement disorder adverse effects among patients with Parkinson's disease whereas quetiapine is not (18). Anecdotally, quetiapine has become the most frequently prescribed antipsychotic for Parkinson's disease psychosis, and our prior work (19) confirms that impression, with two-thirds of treated patients taking quetiapine.
Various facility characteristics were associated with the new starts of study medications, and considerable regional variation was also seen even among the three second-generation antipsychotic medications. These variations may reflect differences in local practices or formulary differences at a Veterans Integrated Service Network level. Such variation also suggests that if alternative medications were associated with outcomes or tolerability in certain patient subgroups, further standardizing practices across facilities and regions may improve care.
Exploratory analyses showed differential relationships over time for a couple of facility characteristics, suggesting that the tendency to selectively prescribe any of these five medications to specific patient groups changed during even a relatively short period of 4.5 years. The differential relationships over the years may be due to certain facilities' being quicker in adapting to new evidence or warnings and may also reflect a lack of more definitive evidence for treating neuropsychiatric symptoms for certain patient groups. Changes in prescribing were not likely a result of findings from efficacy studies given that after the black box warnings there were no studies to our knowledge demonstrating differential efficacy of individual antipsychotics for patients with dementia.
One limitation of our study is that it is based on VHA data. VHA patients are mostly male and have more medical burden than the general population. Because of generous VHA pharmacy coverage, cost is less of a factor in the medication choice in the VHA than in other settings, but there are no data to our knowledge to suggest that VHA providers prescribe differently from non-VHA providers. In addition, in comparison with other national data, we note that there are striking similarities on many key variables that might affect provider antipsychotic prescribing practices (such as race mix and prevalence of key psychiatric and medical conditions) between our data and data from the Aging, Demographics and Memory Study (20,21).
Haloperidol use was associated with not having a psychiatric clinic visit 30 days before medication starts, which may not be a reliable proxy for a nonpsychiatrist's initiating the prescription. A number of these prescriptions may have been started during inpatient hospitalizations given that many patients on haloperidol had delirium. We have no data on provider type during inpatient stays. Nonetheless, we speculate that psychiatrists prefer first-line use of second-generation antipsychotics because the inverse association between prior psychiatric visits and haloperidol use continued after we adjusted for various psychiatric illnesses and prior psychiatric hospitalization. Our study also showed increasing quetiapine use, which may be from increasing use of low-dose quetiapine for evening sedation and hypnotic purposes, and we did not incorporate dose in our analysis. Additional factors that might explain the choice of a particular medication were not available in our administrative data and include dementia severity, type of behavioral symptoms, and various characteristics of prescribing physicians. Changes in prescription practices, in the provision of health care, or in training systems may have also affected the choice of medication, but no known policy changes or regional differences in prescription practices occurred during the period other than the 2005 and 2008 FDA warnings.
Conclusions
In addition to highlighting factors associated with medication choices, our results underscore the importance of addressing selection bias when conducting comparative studies of these medications using observational pharmacoepidemiologic data. Future studies examining risk or outcome differences across these potential alternative medications need to pay attention to the strong patient characteristics shown to be associated with the initial medication choice as well as the differential year-to-year variation in the relationships.
1
2 : Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 294:1934–1943, 2005 Crossref, Medline, Google Scholar
3 : Risk of death in elderly users of conventional versus other psychiatric medications. New England Journal of Medicine 353:2335–2341, 2005 Crossref, Medline, Google Scholar
4 : Antipsychotic drug use and mortality in older adults with dementia. Annals of Internal Medicine 146:775–786, 2007 Crossref, Medline, Google Scholar
5 : Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Canadian Medical Association Journal 176:627–632, 2007 Crossref, Medline, Google Scholar
6 : Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. American Journal of Psychiatry 164:1568–1576, 2007 Link, Google Scholar
7 : Trends in antipsychotic use in dementia 1999–2007. Archives of General Psychiatry 68:190–197, 2011 Crossref, Medline, Google Scholar
8 : A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases 40:373–383, 1987 Crossref, Medline, Google Scholar
9 : Regression Models for Categorical Dependent Variables Using Stata, 2nd ed. College Station, Tex, Stata Press, 2006 Google Scholar
10 : A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica 48:817–838, 1980 Crossref, Google Scholar
11 : Racial disparity in the use of atypical antipsychotic medications among veterans. American Journal of Psychiatry 160:1817–1822, 2003 Link, Google Scholar
12 : Who are patients on conventional antipsychotics?. Schizophrenia Bulletin 29:195–199, 2003 Crossref, Medline, Google Scholar
13 : Mental health service utilization by African Americans and Whites: the Baltimore Epidemiologic Catchment Area Follow-Up. Medical Care 37:1034–1045, 1999 Crossref, Medline, Google Scholar
14 : Persons with depressive symptoms and the treatments they receive: a comparison of primary care physicians and psychiatrists. International Journal of Psychiatry in Medicine 31:41–60, 2001 Crossref, Medline, Google Scholar
15 : Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. American Journal of Geriatric Psychiatry 14:191–210, 2006 Crossref, Medline, Google Scholar
16 : Antipsychotics for delirium. Cochrane Database of Systematic Reviews 2:CD005594, 2007 Medline, Google Scholar
17 : Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 11(suppl 7):14–24, 2006 Crossref, Medline, Google Scholar
18 : Treatment of psychosis in Parkinson's disease: safety considerations. Drug Safety 26:643–649, 2003 Crossref, Medline, Google Scholar
19 : Patterns and trends in antipsychotic prescribing for Parkinson disease psychosis. Archives of Neurology 68:899–904, 2011 Crossref, Medline, Google Scholar
20 : Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the Aging, Demographics and Memory Study. Journal of the American Geriatrics Society 58:330–337, 2010 Crossref, Medline, Google Scholar
21 : Prevalence of depression among older Americans: the Aging, Demographics and Memory Study. International Psychogeriatrics 21:879–888, 2009 Crossref, Medline, Google Scholar
Figures and Tables
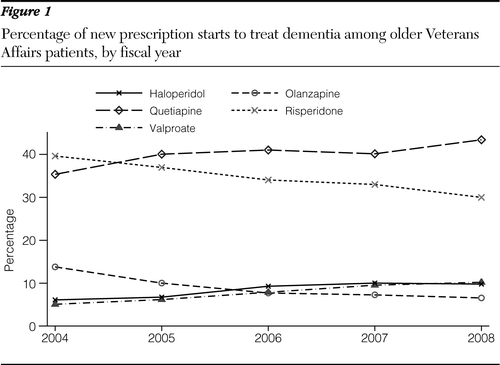
Figure 1 Percentage of new prescription starts to treat dementia among older Veterans Affairs patients, by fiscal year
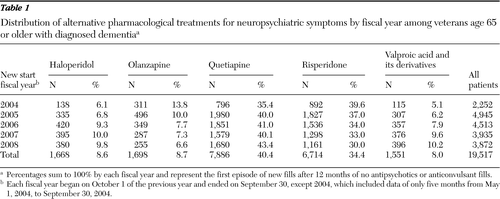
Table 1 Distribution of alternative pharmacological treatments for neuropsychiatric symptoms by fiscal year among veterans age 65 or older with diagnosed dementia
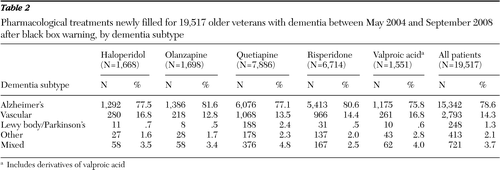
Table 2 Pharmacological treatments newly filled for 19,517 older veterans with dementia between May 2004 and September 2008 after black box warning, by dementia subtype
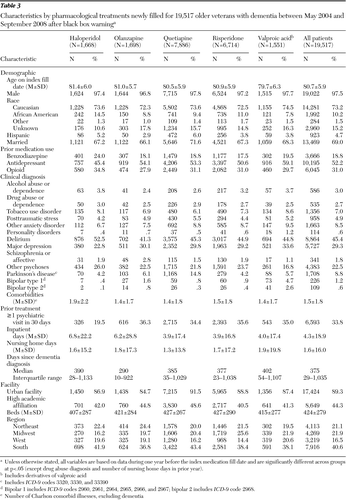
Table 3 Characteristics by pharmacological treatments newly filled for 19,517 older veterans with dementia between May 2004 and September 2008 after black box warning
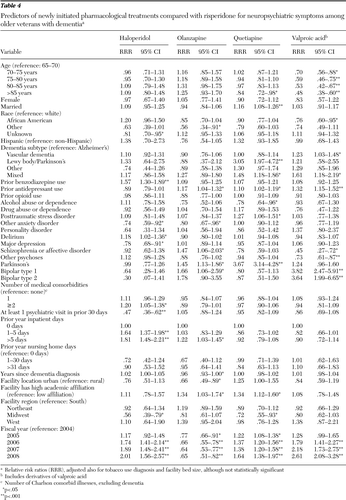
Table 4 Predictors of newly initiated pharmacological treatments compared with risperidone for neuropsychiatric symptoms among older veterans with dementia



