Neuroleptic Malignant Syndrome: A Review
Abstract
OBJECTIVE: Neuroleptic malignant syndrome is an uncommon side effect of antipsychotic medications characterized by severe rigidity, tremor, fever, altered mental status, autonomic dysfunction, and elevated serum creatinine phosphokinase and white blood cell count. This paper presents a concise and comprehensive review of neuroleptic malignant syndrome, written with the practitioner in mind, to provide information that will be useful in actual clinical settings. METHODS: MEDLINE was searched from 1966 to 1997 for key reviews, reports on series of cases of neuroleptic malignant syndrome, individual case reports, and other clinically and theoretically important information. Results and conclusions: Virtually all neuroleptics are capable of inducing the syndrome, including the newer atypical antipsychotics. The standard of care for the recognition of neuroleptic malignant syndrome has shifted considerably over the past 15 years. Neuroleptic malignant syndrome belongs in the differential diagnosis of any patient receiving a neuroleptic who develops a high fever or severe rigidity. In addition to measurement of creatinine phosphokinase and white blood cell count, important tests to rule out other etiologies include urinalysis to measure electrolytes, including calcium and magnesium; kidney, liver, and thyroid function tests; lumbar puncture; an electroencephalogram; and a computed tomography or magnetic resonance imaging scan of the head. Although specific treatment remains controversial, supportive treatment such as antipyretics, a cooling blanket, and intravenous fluids to correct dehydration and electrolyte abnormalities is critical and widely supported by consensus. Most patients recover from neuroleptic malignant syndrome in two to 14 days without any cognitive impairment, and new dysfunction usually is attributable to very high fever, hypoxia, or other complications, rather than neuroleptic malignant syndrome per se.
Neuroleptic malignant syndrome is an uncommon adverse side effect of antipsychotic drugs. The syndrome is characterized by severe rigidity, tremor, fever, altered mental status, autonomic dysfunction, and elevated serum creatinine phosphokinase and white blood cell count. It is a severe, life-threatening condition, and the patient will often appear acutely ill. Diagnosis and treatment of neuroleptic malignant syndrome can be difficult, and the syndrome complicates further psychiatric treatment.
This paper reviews the history and incidence of neuroleptic malignant syndrome and describes risk factors, clinical evaluation, diagnosis, complications, treatment, prevention, and legal issues.
Methods
MEDLINE was searched from 1966 to 1997 for key reviews, reports on series of cases of neuroleptic malignant syndrome, individual case reports, and other clinically and theoretically important information.
A number of excellent reviews on neuroleptic malignant syndrome have been published (2,3,4,5,6,7). The hundreds of case reports and other publications throughout the medical literature in the last 15 years may indicate some uncritical publication. Included in the review presented here are reports of series of cases and individual case reports that provide new information and that offer practical guidance for clinical decision making.
Results
History
Neuroleptics, introduced for clinical use in 1952, were first reported to have caused a syndrome of severe illness by Delay and colleagues (1) in 1960; the term "neuroleptic malignant syndrome" derives from the French "syndrome malin des neuroleptiques" (2). Many case reports of neuroleptic malignant syndrome from many countries have been published since the 1960s, and similar syndromes that were described early in the neuroleptic era may in retrospect have been cases of neuroleptic malignant syndrome that were not termed as such (2).
Incidence and demographic variables
Although neuroleptic malignant syndrome was originally thought to be rare, a proliferation of case reports has created a different impression. Systematic retrospective estimates of incidence vary from .02 to 3.23 percent of psychiatric inpatients receiving neuroleptics (2). This wide variation results from differences in diagnostic criteria for the syndrome, patient populations sampled (for example, acute versus chronic mentally ill), hospital type (for example, state hospital versus university medical center, and treatment style (such as high- versus low-dose neuroleptics), as well as the limitations of retrospective study designs.
Prospective studies in two Boston psychiatric hospitals found incidence rates of neuroleptic malignant syndrome of .07 percent and .9 percent (8,9). More generalizable estimates of incidence will require studies using standardized diagnostic criteria for neuroleptic malignant syndrome with demonstrated validity and reliability in a prospective multicenter design. In any case, the incidence will vary in a given patient population depending on the frequency of risk factors.
Neuroleptic malignant syndrome has been reported among patients of all ages, and about twice as often for men as for women. Most cases have occurred among patients between the ages of 20 and 50, likely paralleling peak neuroleptic use. Patients with suspected neuroleptic malignant syndrome usually have a history of neuroleptic therapy, but not always. Virtually all neuroleptics are capable of inducing the syndrome, including the newer atypical antipsychotics clozapine (10,11,12), risperidone (13,14), and olanzapine (McDaniel K, Evani R, Levenson J, unpublished data, 1998). Although in many of these cases questions remain about the role of comorbid conditions or concomitant medications, it is clear that the atypical antipsychotics are not entirely free of the risk of neuroleptic malignant syndrome. The antiemetic metoclopramide and the tricyclic antidepressant amoxapine have also been reported to cause the syndrome (15,16,17,18,19), presumably because of their dopamine-blocking properties.
Underlying diagnoses most commonly are schizophrenia or affective disorders, but the syndrome also occurs among patients with other conditions for which neuroleptics are used, including dementia, delirium, other psychoses, and mental retardation. Neuroleptic malignant syndrome (or very similar syndromes) has also been reported among patients who have extrapyramidal disorders, such as Parkinson's disease, Wilson's disease, Huntington's chorea, and striatonigral degeneration, and who have received neuroleptics or dopamine-depleting agents or have had dopamine agonists abruptly withdrawn (20,21,22).
Risk factors and clinical evaluation
Both physiologic and environmental factors have been suggested to predispose patients to the development of neuroleptic malignant syndrome. High on the list of conditions suspected to promote the syndrome is dehydration (23,24). Agitation, poor oral intake, and elevated temperature all promote dehydration and hence increase the risk. Patients with prior episodes of neuroleptic malignant syndrome are at higher risk. Receiving high doses of neuroleptics, especially by intramuscular injection, may also be a risk factor (25). However, Lazarus and associates (2) concluded that neuroleptic malignant syndrome appeared not to be dose related, often occurring at standard neuroleptic dosages.
Other suggested risk factors include rapid rate of neuroleptic loading, depot neuroleptics, prolonged use of restraints, use of other medications with neuroleptics (especially lithium), poorly controlled neuroleptic-induced extrapyramidal symptoms, treatment-resistant extrapyramidal symptoms, withdrawal of antiparkinsonian medications, a diagnosis of an affective disorder, alcoholism, organic brain syndrome or previous brain injury, extrapyramidal disorder (for example, Parkinson's disease and Huntington's disease), iron deficiency, and catatonia. Some evidence exists that iron deficiency is a risk factor in a number of movement disorders besides neuroleptic malignant syndrome, such as akathisia and nocturnal myoclonus. However, the association may be more apparent than real (26). Although familial clustering has been reported (27), genetically transmitted risk for neuroleptic malignant syndrome has not been considered clinically significant.
A list of basic and optional examinations for the clinical evaluation of neuroleptic malignant syndrome is provided in Table 1. On physical examination, patients with the syndrome will typically have fever (above 37° C), muscle rigidity, altered consciousness, and autonomic dysfunction. Muscle rigidity that is unresponsive to anticholinergic treatment may be the first sign of neuroleptic malignant syndrome or may be simultaneously identified with increased temperature. Rigidity may range from muscle hypertonicity to severe, lead-pipe rigidity. Parkinsonian findings are common in neuroleptic malignant syndrome, but other movement disorders may be present at the same time (3).
Neurologic dysfunction may include tremors, abnormal reflexes, bradykinesia, chorea, dystonias (including opisthotonos, trismus, blepharospasm, and oculogyric crisis), nystagmus and opsoclonus, dysphagia, dysarthria, aphonia, and seizures.
Altered consciousness may range from a decreased awareness of one's surroundings or confusion to obtundation or total unresponsiveness. Other mental status abnormalities may occur, such as agitation or delirium. Distinguishing between mental abnormalities due to the original psychiatric illness and those due to neuroleptic malignant syndrome can be difficult.
Autonomic dysfunction in neuroleptic malignant syndrome is manifested in a number of findings. Hypertension, postural hypotension, and labile blood pressure are often identified, along with tachycardia and tachypnea when measuring vital signs. Other autonomic disturbances may consist of sialorrhea, diaphoresis, skin pallor, and urinary incontinence. Physical signs of dehydration such as dry mucous membranes, sunken eyes, and increased skin turgor are important to note. Myoglobinuria may be revealed by dark urine that does not contain red blood cells.
In the diagnostic evaluation of suspected neuroleptic malignant syndrome, in addition to taking a careful history and doing a physical examination, physicians will usually find it helpful to obtain a number of laboratory examinations. Creatinine phosphokinase (CPK) levels are often elevated in neuroleptic malignant syndrome secondary to skeletal muscle damage and can reach very high levels (above 100,000 units per liter). CPK may also be elevated by the use of intramuscular injections or restraints, but usually at lower levels (below 600 units per liter) than in neuroleptic malignant syndrome. Serial CPK measurements obtained during treatment of a patient with neuroleptic malignant syndrome will typically show falling levels with resolution of the syndrome. Falling CPK levels but fever spikes correctly led to a search for infection in a patient with neuroleptic malignant syndrome and AIDS (28).
The white blood cell count is often elevated; a range between 10,000 and 40,000/mm3 is commonly reported. A shift to the left in white blood cell count may or may not be found.
In addition to CPK measurement and white blood cell count, important tests to rule out other etiologies of the symptoms include urinalysis to measure electrolytes, including calcium and magnesium; kidney, liver, and thyroid function tests; lumbar puncture; an electroencephalogram; and a computed tomography (CT) scan or magnetic resonance imaging (MRI) scan of the head. Other studies such as measurement of blood gases, coagulation studies, blood cultures, a toxicology screen, and determination of serum lithium level should be done if appropriate. An electromyogram and muscle biopsy are generally not helpful with the diagnosis, often showing only nonspecific changes, if any.
Diagnosis
Diagnostic criteria.
Diagnostic criteria—a somewhat controversial area among investigators—have been proposed for neuroleptic malignant syndrome. Several systems have been suggested to diagnose the syndrome (4,29,30,31). Diagnostic criteria evolved in the literature as case reporting grew and series of cases were reviewed.
Lazarus and associates (2) concluded that "hyperthermia and muscle rigidity are cardinal features of neuroleptic malignant syndrome" and proposed their own set of criteria consisting of treatment with neuroleptics within seven days before onset of the syndrome, hyperthermia, muscle rigidity, the exclusion of systemic or neuropsychiatric illness that could account for the syndrome, and three of the following: change in mental status, tachycardia, change in blood pressure, tachypnea, CPK elevation or myoglobinuria, leukocytosis, and metabolic acidosis.
Patients believed to be in the early stages of neuroleptic malignant syndrome have been described, as have patients with mild forms that did not develop into the full-blown syndrome—some despite continued neuroleptic therapy (32,33,34). Neuroleptic malignant syndrome may manifest along a symptom spectrum and a severity spectrum. At one end of the symptom spectrum, only two of the cardinal signs among the many features of neuroleptic malignant syndrome may be manifest. On the severity spectrum, symptoms may be mild, moderate, or severe. When all the manifestations occur together or in quick succession and are moderate to severe, the diagnosis would be easy, and consensus would be obtained. However, if symptoms are limited to two and are mild (for example, rigidity and fever), considerable debate may occur about whether the patient has neuroleptic malignant syndrome.
Likewise, clozapine-related neuroleptic malignant syndrome may not present with typical extrapyramidal rigidity. Among these "spectrum" cases, we are not at the point of being able to say who will develop full-blown neuroleptic malignant syndrome. The risk of morbidity and even mortality deserves serious consideration. Withdrawing the offending neuroleptic is still advisable if early neuroleptic malignant syndrome is suspected.
Differential diagnosis.
The majority of patients receiving neuroleptics who develop fever and rigidity will have conditions other than neuroleptic malignant syndrome, making differential diagnosis of prime importance. Although active steps must be taken to rule out other conditions, neuroleptic malignant syndrome should not be regarded entirely as a diagnosis of exclusion because of the importance of promptly withholding neuroleptics when the syndrome is suspected. At the same time, physicians must be careful not to conclude prematurely that the diagnosis is neuroleptic malignant syndrome for patients who may have a medical cause for a fever, such as aspiration pneumonia, superimposed on neuroleptic-induced extrapyramidal symptoms (35).
A very large number of conditions could be considered in the differential diagnosis of neuroleptic malignant syndrome if each sign, particularly fever or catatonia, were examined individually. Here we focus on conditions that may present with several or even all the signs of neuroleptic malignant syndrome. These conditions are shown in Table 2.
Malignant hyperthermia is a hypermetabolic state of skeletal muscle most frequently associated with the administration of halogenated inhalation anesthetic agents and succinylcholine. Originally thought to result from an autosomal dominant trait, malignant hyperthermia is now considered to have a multifactorial pattern of inheritance. The clinical presentation is identical to neuroleptic malignant syndrome, and rhabdomyolysis and death are common outcomes. Intravenous dantrolene sodium is specifically therapeutic in treatment of malignant hyperthermia, and oral dantrolene is an effective preoperative prophylaxis.
The diagnosis of malignant hyperthermia (or of the trait) is reliably established by exposing biopsied muscle tissue to caffeine or halothane in vitro, which results in a hypercontractile response when compared with normal muscle. Muscle tissue from patients with neuroleptic malignant syndrome does not demonstrate a hypercontractile response to caffeine or halothane. In most cases neuroleptic malignant syndrome and malignant hyperthermia can be distinguished clinically by the different settings and drug exposures. In addition, no family histories of hyperthermia have been documented in patients with neuroleptic malignant syndrome.
Lethal catatonia is a syndrome in which mutism, extreme motor excitement, clouding of consciousness, and fever may progress to severe autonomic disturbances, stupor and coma, and death. Mann and colleagues (36) have suggested that it should be regarded as a final common pathway for a variety of psychiatric and medical illnesses. They conceptualize neuroleptic malignant syndrome as one (iatrogenic) cause of lethal catatonia. Others regard functional lethal catatonia and neuroleptic malignant syndrome as separate entities that can be clinically differentiated, pointing out that, among other differences, lethal catatonia begins with extreme psychotic excitement, whereas neuroleptic malignant syndrome begins with severe muscle rigidity (37). Whatever the relationship is between neuroleptic malignant syndrome and lethal catatonia, in practice the distinction may be very difficult if not impossible to make (38). In any case, neuroleptics should be discontinued because they are usually ineffective in lethal (or severe) catatonia (2,36).
Neuroleptics may cause other adverse effects that may be confused with neuroleptic malignant syndrome. Heat stroke is a risk because neuroleptics suppress central heat loss mechanisms, resulting in increased vulnerability to hot environments or marked exertion. Although hot environmental conditions may increase the risk for neuroleptic malignant syndrome (39), most cases of the syndrome have occurred under normal temperature conditions. Heatstroke is also distinguished by hot, dry skin, rather than the diaphoresis in neuroleptic malignant syndrome, and by the absence of rigidity.
Neuroleptics can cause severe extrapyramidal symptoms, even resulting in rhabdomyolysis (4) or catatonia (40), but such patients do not manifest marked fever, leukocytosis, or autonomic disturbances unless a secondary complication has developed, such as infection or pulmonary embolus. Causes of rhabdomyolysis, other than neuroleptic malignant syndrome or severe dystonia, that occur frequently among severely ill psychiatric patients include immobilization, the use of restraints, dehydration, malnutrition, multiple intramuscular injections, alcoholism, and trauma.
Central nervous system infections including meningitis, encephalitis, or neurosyphilis may mimic neuroleptic malignant syndrome. Lumbar puncture and an electroencephalogram should lead to the distinction. Except for a few cases of slightly elevated protein in cerebrospinal fluid, the cerebrospinal fluid of patients with neuroleptic malignant syndrome does not demonstrate the changes in glucose, white cells, or protein expected in central nervous system infections. A CT or MRI scan is helpful in ruling out brain abscesses and other structural lesions.
Allergic drug reactions may produce fever and autonomic instability but not rigidity. Signs of allergy should be looked for, including rash, wheezing, urticaria, and eosinophilia. Various toxic encephalopathies may resemble neuroleptic malignant syndrome, including those due to strychnine, tetanus, botulism, anticholinergic delirium, lithium toxicity, and hyperthermia induced by other psychotropic drugs such as tricyclic antidepressants, monoamine oxidase inhibitors, stimulants, and hallucinogens (2). Hyperthyroidism and hypocalcemic or hypomagnesemic tetany must also be considered and are easily tested for. Parkinson's disease and other neurologic disorders may include rigidity, trauma, and autonomic neuropathy but not leukocytosis, fever, or elevated CPK.
A diagnosis of neuroleptic malignant syndrome must be considered before specific treatments of the syndrome can be implemented. Thorough evaluation as suggested should enable the clinician to rule out the many neurologic, toxic, infectious, or metabolic causes with similar clinical pictures. A workup also serves to identify possible concurrent medical illnesses and complications of neuroleptic malignant syndrome.
Complications
As implied by the word "malignant," death may occur as a result of neuroleptic malignant syndrome or its life-threatening complications. Levinson and Simpson (35) reported that 79 percent of the patients in their sample with neuroleptic malignant syndrome made a full recovery, and 8 percent died. In a review of 202 case reports, Shalev and associates (7) noted a decreasing mortality—11.6 percent since 1984 and 25 percent before 1984—probably attributable to better recognition of the syndrome with earlier intervention. In their review, patients with neuroleptic malignant syndrome who had organic mental disorders had significantly higher mortality than patients with functional psychoses, as did patients who developed myoglobinuria and renal failure.
Complications of neuroleptic malignant syndrome are often physiologic consequences of severe rigidity and the immobilization that comes with it. Poor oral intake leads to dehydration, increasing the risk of rhabdomyolysis, which in turn may lead to acute renal failure. Deep venous thrombosis and pulmonary embolism may occur as another consequence of rigidity, immobilization, and dehydration and accounts for about a fourth of fatalities in neuroleptic malignant syndrome (41). Difficulty swallowing combined with altered mental status may lead to aspiration and pneumonia, and patients may need to be intubated and receive ventilatory support. Other causes of pulmonary failure include adult respiratory distress syndrome (especially with rhabdomyolysis) and shock lung (42).
Many other serious complications of neuroleptic malignant syndrome or its treatment have been reported, such as myocardial infarction, disseminated intravascular coagulation, and sepsis (4). Cerebellar neuronal degeneration has been attributed to hyperpyrexia from neuroleptic malignant syndrome (43). Persons with lithium in their system during neuroleptic malignant syndrome, even at nontoxic levels, may be particularly at risk for cerebellar damage and ataxia from hyperthermia (2). Indeed, a range of persistent neurological abnormalities after resolution of neuroleptic malignant syndrome is possible, including neuropsychological (cognitive) impairments (44). Most patients recover from neuroleptic malignant syndrome without any cognitive impairment, and new dysfunction usually is attributable to very high fever, hypoxia, or other complications, rather than to neuroleptic malignant syndrome per se.
Treatment
As we have mentioned repeatedly, neuroleptic medication must be stopped as soon as neuroleptic malignant syndrome is suspected. It is the most critical and definitive intervention. We have been consulted in several cases in which the treating physician had doubts about the diagnosis and awaited our opinion before stopping neuroleptic medication. In some cases, such a delay may lead to prolongation of the episode.
Discontinuation of lithium is recommended. Discontinuation of anticholinergics or substitution with a dopamine agonist should be considered in case of residual extrapyramidal symptoms or an upsurge of parkinsonian symptoms. Dopamine agonist medications such as amantadine should be continued if already in use, as their withdrawal may worsen neuroleptic malignant syndrome.
After recognition of the syndrome and discontinuation of neuroleptics, the usual clinical course of neuroleptic malignant syndrome runs two to 14 days (2,6), and medications and other therapies are gradually withdrawn while recovery is monitored. Prolonged cases have occurred, particularly among patients who received long-acting preparations (45,46). In both of our most recently encountered cases in which neuroleptic malignant syndrome occurred after depot neuroleptic administration, the complete resolution of symptoms took more than 28 days from initial hospitalization and more than 35 days from the time of the last injection.
Treatment setting.
Most cases meeting the full criteria for neuroleptic malignant syndrome should be treated in a medical intensive care unit. Unless the signs of neuroleptic malignant syndrome are questionable, patients referred to neurology or psychiatry services should be transferred to the intensive care unit for initial care. However, criteria for keeping a patient with suspected neuroleptic malignant syndrome on a psychiatry service could include vital signs that are only slightly elevated or that respond rapidly to minor measures, such as antipyretics; the patient's ability to take fluids orally; normal cardiorespiratory function; normal renal function; and, possibly, a CPK level of less than 1,000 units per liter.
We recommend that after several criteria are met, the patient should be transferred to a medical-psychiatry service or a general psychiatry service with experience in dealing with neuroleptic malignant syndrome. The transfer should take place after vital signs have normalized, dehydration and any electrolyte imbalance have been corrected, normal cardiorespiratory function exists, no evidence of renal failure exists, and at least two consecutive CPK measurements show a falling trend. Inpatient care is recommended until CPK levels have normalized, vital signs are persistently normal, and any psychiatric condition is stable and permits discharge. These criteria suggest that the complete clearance of the neuroleptic may be necessary before full recovery occurs.
The severe and prolonged morbidity experienced by such patients underscores the importance of correct diagnosis of the psychiatric disorder, careful consideration of all options before deciding on a depot neuroleptic, and use of the lowest dose needed. Frequent dosing of the depot preparation beyond that recommended by the manufacturer or substantiated by published research is discouraged.
Supportive therapies.
Patients will require antipyretics, a cooling blanket, and intravenous fluids to correct dehydration and electrolyte abnormalities. Treatment with a short-term antihypertensive, such as nifedipine, and oxygen may be required. Some patients require intubation and ventilator support. The gag reflex may be lost in neuroleptic malignant syndrome, which can lead to aspiration pneumonia. It is important to check for this reflex, provide parenteral nutrition until it returns, and position the patient appropriately to avoid aspiration.
Subcutaneous heparin is recommended to guard against deep venous thrombosis and pulmonary embolism. Dialysis may be needed if renal failure develops, but dialysis is ineffective for removing neuroleptics as they are strongly protein bound. Prolonged muscular rigidity is not uncommon in neuroleptic malignant syndrome, and regular physical therapy is indicated. Decubitus ulcers and brachial and other neuropathies must also be guarded against in treating patients with neuroleptic malignant syndrome.
Attention to nutritional support is important because most patients cannot eat due to altered mental status or rigidity with esophageal spasm, and many may have already been malnourished before developing the condition. Neuroleptic malignant syndrome is a very stressful syndrome, particularly because of increased body temperature and the energy expenditure of prolonged rigidity, and good nutrition may help minimize rhabdomyolysis and other tissue damage. Neuroleptic malignant syndrome may also trigger ketoacidosis in diabetics. Thus the complete management of a patient with the syndrome will include a review of all comorbid medical disorders and appropriate therapy.
Drug therapy.
It is important to assess the risks and benefits of drug treatment before initiating pharmacotherapy for neuroleptic malignant syndrome. We recommend starting supportive care first and observing the course and severity of the syndrome. If the patient's condition does not show a trend toward improvement or worsens, additional pharmacologic interventions should be considered. In cases of worsening symptoms—increasing rigidity, increasing CPK levels, and persistent high temperature—medications should not be withheld. A stable condition, minimal fluctuations, or actual improvement would argue for a "wait-and-see" approach.
Usually, the decision to use or not use specific therapies can be made in one to three days of observation and support. The patient may benefit from supportive treatment alone without the risk of further morbidity. In other words, the temptation to rush into drug treatment should be avoided.
A number of somatic treatments have been tried for neuroleptic malignant syndrome, with mixed results. The dopamine agonists bromocriptine, amantadine, apomorphine, lisuride, and carbidopa-levodopa have a theoretical and clinical rationale in the treatment of the syndrome, and many case reports support their use. Bromocriptine may be the preferred choice among these dopamine agonists (2). We recommend starting with 2.5 mg orally two to three times a day. If needed, the total dose should be increased by 2.5 to 7.5 mg daily, up to a total of 45 mg a day. The patient should be monitored for adverse effects such as nausea, vomiting, psychosis, and alterations in mental status.
Dantrolene, a muscle relaxant, has been used to treat neuroleptic malignant syndrome based on its efficacy in malignant hyperthermia. Dantrolene is specifically recommended for severe hyperthermia, which it may relieve by relaxing skeletal muscle. Dantrolene can cause a drug-induced hepatitis, and liver function tests should be checked during its use. The simultaneous use of bromocriptine and dantrolene for an individual with neuroleptic malignant syndrome may be warranted. Dantrolene can be given by intravenous bolus, 1 to 10 mg per kilogram of body weight, or by divided oral doses of 50 to 600 mg a day (2).
Because of the absence of controlled studies of drug therapy for neuroleptic malignant syndrome, the reported effectiveness of specific drug treatment for the average case may be illusory. Rosenberg and Green (47) found the "addition of dantrolene or bromocriptine significantly shortened the time to clinical response," while a prospective series of 24 cases did not find bromocriptine or dantrolene markedly important in bringing about improvement (48). A case control analysis of 734 cases by Sakkas and colleagues (49) concluded that dantrolene, bromocriptine, and amantidine seemed to be the most effective in treating neuroleptic malignant syndrome.
Other somatic treatments tried with mixed results include benzodiazepines, barbiturates, verapamil, and curare. Electroconvulsive therapy (ECT) has received much attention as a treatment for neuroleptic malignant syndrome, with good results both for the syndrome and for the underlying psychiatric condition (50,51,52). For example, Scheftner and Shulman (51) noted that 26 of 31 patients with neuroleptic malignant syndrome given ECT had a good response. Davis and colleagues (52) found that specific drug or somatic therapy may reduce mortality.
The possibility that iron deficiency aggravates several movement disorders including neuroleptic malignant syndrome has led some to recommend iron supplementation as part of standard treatment. This treatment should be reserved for patients with demonstrated deficiency, since indiscriminate iron supplementation has its own risks (53).
Retreatment of the primary illness
Psychosis itself is a severe illness with a significant risk of mortality, and restarting treatment with neuroleptics for a patient who has experienced neuroleptic malignant syndrome may be necessary because of continued psychiatric illness (54). However, before antipsychotic therapy is resumed, nonneuroleptic therapies, such as lithium, carbamazepine, benzodiazepines (lorazepam and clonazepam), and ECT, should first be considered.
Should restarting a neuroleptic be deemed necessary, the physician should switch to a neuroleptic in a different chemical class and with a lower D2 affinity than the one that produced the neuroleptic malignant syndrome. The availability of the serotonin-dopamine antagonist antipsychotics (atypical antipsychotics), which possess only moderate affinity for the nigrostriatal D2 , has clearly increased the options available to the clinician.
However, we again note that these atypical agents are capable of inducing neuroleptic malignant syndrome (10,11,12,13,14; McDaniel K, Evani R, Levenson J, unpublished data, 1998). Based on case report literature and the pharmacological profile of clozapine, we recommend clozapine as a preferred antipsychotic in the rechallenge of patients for whom a neuroleptic is essential. Of course, clozapine exposes the patient to risks such as seizure and agranulocytosis.
Monitoring vital signs and CPK level is advisable to identify relapse early and to stop the neuroleptic as soon as possible. In any case, retreatment with neuroleptics should be reserved for those patients with a clear-cut psychosis. A discussion with the patient and his or her family of the risks and benefits of retreatment is advisable, and obtaining informed consent may be prudent.
A review of 41 cases of neuroleptic therapy following resolution of neuroleptic malignant syndrome reported the risk of relapse was less if at least five days passed from recovery to rechallenge (55). No relationship was found between relapse and patients' age or sex or the drug used. Susman and Addonizio (56) suggested that the use of high-potency neuroleptics is possibly a risk factor for recurrence and also stressed the need for complete recovery from neuroleptic malignant syndrome before reintroduction of a neuroleptic. Rosebush and coworkers (57) recommended a minimum of two weeks after recovery from neuroleptic malignant syndrome before reintroduction of neuroleptics, and they found potency and dosage less important than the time factor. Lazarus and associates (2) concluded in their review that "recurrences appear to correlate more closely with the dopamine antagonist potency of the drug involved."
Although fatal recurrences have been reported (34), neuroleptics can be reintroduced safely with monitoring. After discharge from a hospital, patients' long-term outcome after neuroleptic malignant syndrome can be good, even with continued neuroleptic therapy for the primary psychiatric illness (58).
Prevention
Decreasing the putative risk factors for neuroleptic malignant syndrome includes early detection and treatment of neuroleptic-induced side effects. Because the side effects of neuroleptics vary among patients, strategies to avoid rigidity are stressed. Avoidance of dehydration, the use of physical restraints for long periods, and numerous intramuscular injections are other suggested measures to decrease risk. Temperature should be closely monitored in agitated or restrained patients.
The very significant role that such cofactors may play in precipitating neuroleptic malignant syndrome was emphasized by a longitudinal follow-up study of patients with the syndrome conducted by Pope and colleagues (59). Fostering nutrition along with hydration is also recommended. We recommend that patients with a history of neuroleptic malignant syndrome not be given depot neuroleptics.
Early recognition of the syndrome can limit morbidity, but the clinician must have an index of suspicion for early recognition. Gelenberg and associates (60) stressed the importance of screening for a history of neuroleptic malignant syndrome among patients being readmitted to a hospital for psychiatric treatment. It has also been suggested that vulnerability to the syndrome may be familial (27), and a family history of neuroleptic malignant syndrome should be heeded. Empirical evidence exists that instituting a preventive program will reduce the incidence of neuroleptic malignant syndrome (61). Some psychiatrists and anesthesiologists remain fearful about anesthetizing patients who have a history of the syndrome; however, it has now been well documented that patients with such as history are at no extra risk from anesthesia (50,58).
Evidence is accumulating that rapidly switching patients from clozapine to risperidone may pose a risk of severe withdrawal effects, including those stemming from serotonergic and cholinergic rebound. We have empirically based concerns, based on two recent cases, that a window of risk exists for neuroleptic malignant syndrome during rapid switch from atypical neuroleptic to typical neuroleptic. We recommend a very gradual switch.
The biology of neuroleptic malignant syndrome
The pathophysiology of neuroleptic malignant syndrome is not fully understood. Massive and sudden reduction in dopaminergic activity secondary to neuroleptic-induced dopamine blockade is considered the chief mechanism mediating the symptoms of the syndrome. Imbalance between dopaminergic activity and those of other transmitters such as gamma amino butyric acid (GABA) and acetylcholine have also been proposed. Some evidence implicates a hypernoradrenergic state. The dopamine blockade theory remains the most viable and accepted theory, with the involvement of both striatal and hypothalamic dopamine receptor blockade (40). In addition to epidemiological data from case reports, several other lines of evidence, including studies of monoamines in cerebrospinal fluid, support the involvement of dopaminergic blockade in neuroleptic malignant syndrome, and there is not much controversy about this involvement.
However, current neurochemical theories do not explain why neuroleptic malignant syndrome occurs in a very small number of patients in the large population who receive neuroleptics. Other factors critical to initiating the syndrome or facilitating the progression of isolated symptoms have to be invoked to explain its idiosyncratic occurrence. The muscle rigidity seen in neuroleptic malignant syndrome does not respond well to the usual anticholinergic drugs, unlike that seen in drug-induced parkinsonism. It is possible either that the hyperthermia perpetuates rigidity and renders these drugs ineffective or that the rigidity seen in neuroleptic malignant syndrome is qualitatively different—which is another reason to look for the role of other factors. Such cofactors might include genetic predisposition, environmental injury, and iatrogenic pharmacological insult. Few studies have been designed to address or define the specificity of such factors.
In a case-control study, Keck and associates (25) confirmed that psychomotor agitation, dosage of neuroleptic, number of intramuscular injections, and rate of increase of the neuroleptic dosage were all significant factors associated with neuroleptic malignant syndrome. The exact manner in which these factors operate remains to be established. It is also evident from the case report literature that none of them is always necessary for the syndrome to be induced.
Another question in understanding the pathophysiology of neuroleptic malignant syndrome is the sequence in which the various components of the syndrome develop. For example, if muscular rigidity is the first sign, extrapyramidally mediated central nervous system dopaminergic dysfunction can account for it. Subsequent hyperthermia could be secondary to muscle-related peripheral mechanisms of heat generation. On the other hand, if the rise in temperature were the first manifestation, endogenous central thermodysregulation or exogenous factors such as dehydration and excessive motor activity, which is often seen among patients before they develop neuroleptic malignant syndrome, might cause the hyperthermia. Hyperthermia is believed to enhance neuroleptic binding to the dopamine receptors (62), which may cause the excessive rigidity.
Addonizio and colleagues (6) concluded from their retrospective review of cases of neuroleptic malignant syndrome that for most patients extrapyramidal symptoms occur before a rise in temperature, suggesting that muscle contraction is a factor in hyperthermia. Mental changes and extrapyramidal symptoms were also found to be the initial manifestations in another review of 153 cases of neuroleptic malignant syndrome (63). Further prospective study is needed and should include examination of the course and outcome of patients who develop incomplete forms of the syndrome. A better understanding of the pathophysiological sequence of the syndrome could enlighten pharmacotherapy.
In view of the clinical similarities between malignant hyperthermia and neuroleptic malignant syndrome, the question of whether a primary skeletal muscle pathology is present in neuroleptic malignant syndrome has been investigated. Conflicting results have been found. Some investigators have found abnormal contractile response of muscle tissue to halothane and fluphenazine from subjects with neuroleptic malignant syndrome (64,65) while others have not (66,67,68).
It is unlikely that neuroleptic malignant syndrome is a variant of malignant hyperthermia, given the safety with which patients with neuroleptic malignant syndrome have received anesthesia and the safety with which patients with malignant hyperthermia have received neuroleptics. Phenothiazines are well known to produce complex effects on muscle, including displacement of membrane-bound calcium ions, antagonism of calmodulin, uncoupling of mitochondrial oxidation, and perturbation of glucose and cholesterol metabolism (3). These mechanisms may explain the rhabdomyolysis noted in severe neuroleptic malignant syndrome.
It should be clear from this discussion that ample opportunity and need exist for future research. Significant advances have been made in pharmacogenetics, and it may be appropriate to examine whether there are allelic variations of polymorphisms in the dopamine receptor family to explain the idiosyncratic occurrence of neuroleptic malignant syndrome. A prospective and large multicenter study with at least four treatment arms—supportive care, supportive care plus bromocriptine, supportive care plus dantrolene, and supportive care plus a combination of bromocriptine and dantrolene—could help establish a standard for treatment.
Another area of treatment research is whether to re-treat patients who have recovered from neuroleptic malignant syndrome with an antipsychotic rather than adjunct medications and how long one should wait before initiating the new therapy. Related to the role of atypical antipsychotics is the question of whether different mechanisms of action pose an advantage for the acute patient recovering from neuroleptic malignant syndrome or open a window of further vulnerability.
Legal issues
Although the legal issues surrounding neuroleptic malignant syndrome are not unique, they are worthy of careful consideration. Malpractice litigation about neuroleptic malignant syndrome is quite common, although little attention has been paid to it in the literature (69,70,71). The dramatic symptom picture, the associated morbidity and mortality, and the name of the syndrome itself all seem to draw the interest of plaintiffs' attorneys. As one would expect, lawsuits involving neuroleptic malignant syndrome range from those that appear justified to the spurious and frivolous.
For example, a justified lawsuit would involve continued administration of neuroleptics, especially by intramuscular injection, despite the development of full-blown symptoms of neuroleptic malignant syndrome, even after a consultant's suggestion that the diagnosis should be considered. At the other end of the spectrum, some attorneys and their expert witnesses see neuroleptic malignant syndrome lurking behind any catastrophic outcome for a psychotic patient receiving neuroleptics, particularly if fever or rigidity have occurred.
Physicians have been sued for not using dantrolene or bromocriptine to treat neuroleptic malignant syndrome, or for not using them "quickly enough" or in "sufficiently high doses" in the opinion of the plaintiff's expert witness. Other litigation has questioned the use of neuroleptics for patients with a history of neuroleptic malignant syndrome or similar symptoms.
For clinical as well as risk management reasons, we would advise the following steps (shown in Table 3) whenever neuroleptic malignant syndrome is considered a possibility. Seek early consultation from psychiatric, medical, and neurological colleagues. Document a broad differential diagnosis and rationally narrow it down. Whenever neuroleptic malignant syndrome is seriously under consideration, it is best to consider withholding neuroleptics, keeping in mind that the risks of continuing neuroleptics must be weighed against the risks of withholding them (the patient's danger to self and others). Temporarily withholding neuroleptics is facilitated when other medication (for example, lorazepam) is effective as a short-term substitute. Given the circumstances under which neuroleptic malignant syndrome develops, the clinician may feel too busy to "worry about the paperwork," but documentation of one's thinking will reduce liability risk and need not be tediously detailed.
Although patients with mild symptoms suggestive of possible neuroleptic malignant syndrome may be appropriately managed in psychiatric settings, more seriously ill patients should be transferred out of freestanding psychiatric hospitals to general hospitals where expertise in psychiatry, neurology, and critical care is available (71). Informing families during or after an episode of neuroleptic malignant syndrome, as well as patients who have sufficiently recovered, is also an important step in eliminating the misunderstandings that are the breeding ground for future lawsuits.
Are physicians prescribing neuroleptics obligated to inform patients about the risk of neuroleptic malignant syndrome? Acutely psychotic patients are often incapable of sufficient understanding. Because neuroleptic malignant syndrome is rare and its onset unpredictable, the value of warning patients about neuroleptic malignant syndrome is questionable. It would be more sensible to inform patients about the importance of reporting high fever, rigidity, or abnormal movements.
Physicians who serve as expert witnesses in cases in which mismanagement is alleged should retain objectivity and humility, eschewing "20/20 hindsight." No test can definitely rule in or rule out neuroleptic malignant syndrome. In our opinion, no proven specific treatment exists for the syndrome despite statements in the literature to the contrary. Experts are not unanimous about the value of dantrolene, bromocriptine, and other specific treatments. There is no way to validly predict which patients will develop a first or recurrent episode of neuroleptic malignant syndrome. On the other hand, known risk factors should have been heeded and neuroleptic malignant syndrome should have been recognized if the symptoms were sufficiently severe.
Conclusions
The standard of care for the recognition of neuroleptic malignant syndrome has shifted considerably over the past 15 years, with literally hundreds of case reports and reviews in the medical literature. Neuroleptic malignant syndrome belongs in the differential diagnosis of any patient receiving a neuroleptic who develops a high fever or severe rigidity. Finally, while specific treatment remains controversial, supportive treatment—for example, rapid cooling for extremely high fever, hydration, and anticoagulation—is critical and is widely supported by consensus.
Dr. Pelonero is associate professor of psychiatry and Dr. Levenson and Dr. Pandurangi are professors of psychiatry in the department of psychiatry at Virginia Commonwealth University-Medical College of Virginia, 1200 East Broad Street, P.O. Box 980710, Richmond, Virginia 23298-0710 (e-mail, [email protected]).
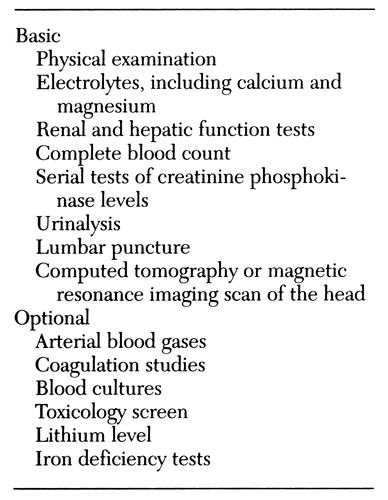 |
Table 1. Basic and optional examinations for clinical evaluation of possible neuroleptic malignant syndrome
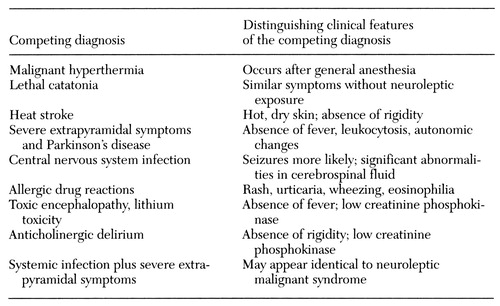 |
Table 2. Differential diagnosis of neuroleptic malignant syndrome
 |
Table 3. Risk management in neuroleptic malignant syndrome
1. Delay J, Pichot P, Lemperiere T, et al: Un neuroleptique majeur non phenothiazine et non reserpine, l'haloperidol, dans le traitement des psychoses [Haloperidol, a nonphenothiazine, nonreserpine neuroleptic for the treatment of psychosis]. Annales Medico-Psychologiques 118:145-152, 1960Medline, Google Scholar
2. Lazarus A, Mann SC, Caroff SN: The Neuroleptic Malignant Syndrome and Related Conditions. Washington, DC, American Psychiatric Press, 1989Google Scholar
3. Kurlan R, Hamill R, Shoullson I: Neuroleptic malignant syndrome. Clinical Neuropharmacology 7:109-120, 1984Crossref, Medline, Google Scholar
4. Levenson JL: Neuroleptic malignant syndrome. American Journal of Psychiatry 142:1137-1145, 1985Link, Google Scholar
5. Pearlman CA: Neuroleptic malignant syndrome: a review of the literature. Journal of Clinical Psychopharmacology 6:257-273, 1986Crossref, Medline, Google Scholar
6. Addonizio G, Susman VL, Roth SD: Neuroleptic malignant syndrome: review and analysis of 115 cases. Biological Psychiatry 22:1004-1020, 1987Crossref, Medline, Google Scholar
7. Shalev A, Hermesh H, Munitz H: Mortality from neuroleptic malignant syndrome. Journal of Clinical Psychiatry 50:18-25, 1989Medline, Google Scholar
8. Gelenberg AJ, Bellinghausen B, Wojcik JD, et al: A prospective survey of neuroleptic malignant syndrome in a short-term psychiatric hospital. American Journal of Psychiatry 145:517-518, 1988Link, Google Scholar
9. Keck PE, Sebastianelli J, Pope HG, et al: Frequency and presentation of neuroleptic malignant syndrome in a state psychiatric hospital. Journal of Clinical Psychiatry 50:352-355, 1989Medline, Google Scholar
10. Pope HG, Cole JO, Choras PT, et al: Apparent neuroleptic malignant syndrome with clozapine and lithium. Journal of Nervous and Mental Disease 174:493-495, 1986Crossref, Medline, Google Scholar
11. Muller T, Becker T, Fritze J: Neuroleptic malignant syndrome after clozapine plus carbamazepine (letter). Lancet 2:1500, 1988Crossref, Medline, Google Scholar
12. Sachdev P, Kruk J, Kneebone M, et al: Clozapine-induced neuroleptic malignant syndrome: review and report of new cases. Journal of Clinical Psychopharmacology 15:365-371, 1995Crossref, Medline, Google Scholar
13. Dave M: Two cases of risperidone-induced neuroleptic malignant syndrome (ltr). American Journal of Psychiatry 152:1233-1234, 1995Medline, Google Scholar
14. Singer S, Richards C, Boland RJ: Two cases of risperidone-induced neuroleptic malignant syndrome (ltr). American Journal of Psychiatry 152:1234, 1995Medline, Google Scholar
15. Friedman LS, Weinrauch LA, D'Elia JA: Metoclopramide-induced neuroleptic malignant syndrome. Archives of Internal Medicine 147:1495-1497, 1987Crossref, Medline, Google Scholar
16. Patterson JF: Neuroleptic malignant syndrome associated with metoclopramide. Southern Medical Journal 81:674-675, 1988Crossref, Medline, Google Scholar
17. Brower RD, Dreyer CF: Neuroleptic malignant syndrome in a child treated with metoclopramide for chemotherapy-related nausea (ltr). Journal of Child Neurology 4:230-232, 1989Crossref, Medline, Google Scholar
18. Madakasira S: Amoxapine-induced neuroleptic malignant syndrome. Drug Intelligence and Clinical Pharmacy 23:50-51, 1989Google Scholar
19. Taylor NE, Schwartz HI: Neuroleptic malignant syndrome following amoxapine overdose. Journal of Nervous and Mental Disease 176:249-251, 1988Crossref, Medline, Google Scholar
20. Friedman JH, Feinberg SS, Feldman RG: A neuroleptic malignant-like syndrome due to levodopa therapy withdrawal. JAMA 254:2792-2795, 1985Crossref, Medline, Google Scholar
21. Gibb WRG: Neuroleptic malignant syndrome in striatonigral degeneration. British Journal of Psychiatry 153:254-255, 1988Crossref, Medline, Google Scholar
22. Kontaxakis V, Stefanis C, Markidis M, et al: Neuroleptic malignant syndrome in a patient with Wilson's disease (ltr). Journal of Neurology, Neurosurgery, and Psychiatry 51:1001-1002, 1988Crossref, Medline, Google Scholar
23. Itoh H, Ohtsuka N, Ogita K, et al: Malignant neuroleptic syndrome: its present status in Japan and clinical problems. Folia Psychiatrica et Neurologica Japonica 31:565-576, 1977Google Scholar
24. Harsch HH: Neuroleptic malignant syndrome: physiological and laboratory findings in a series of nine cases. Journal of Clinical Psychiatry 48:328-333, 1987Medline, Google Scholar
25. Keck PE, Pope HG, Cohen BM, et al: Risk factors for neuroleptic malignant syndrome. Archives of General Psychiatry 46:914-918, 1989Crossref, Medline, Google Scholar
26. Treloar AJ, Crook MA, Tuff P, et al: Iron status, movement disorders, and acute phase response in elderly psychotic patients. Journal of Neurology, Neurosurgery, and Psychiatry 57:208-210, 1994Crossref, Medline, Google Scholar
27. Otani K, Horiuchi M, Kondo T, et al: Is the predisposition to neuroleptic malignant syndrome genetically transmitted? British Journal of Psychiatry 158:850-853, 1991Google Scholar
28. Burch EA, Montoya J: Neuroleptic malignant syndrome in an AIDS patient (ltr). Journal of Clinical Psychopharmacology 9:228-229, 1989Crossref, Medline, Google Scholar
29. Addonizio G, Susman VL, Roth SD: Symptoms of neuroleptic malignant syndrome in 82 consecutive inpatients. American Journal of Psychiatry 143:1587-1590, 1986Link, Google Scholar
30. Roth SD, Addonizio G, Susman VL: Diagnosing and treating neuroleptic malignant syndrome (ltr). American Journal of Psychiatry 143:673, 1986Link, Google Scholar
31. Pope HG, Keck PE Jr, McElroy SL: Frequency and presentation of neuroleptic malignant syndrome in a large psychiatric hospital. American Journal of Psychiatry 143:1227-1233, 1986Link, Google Scholar
32. VanPutten T, Beckson M, Marder SR: The neuroleptic malignant syndrome manifested as a prolonged confusional state (ltr). Journal of Clinical Psychopharmacology 8:229-230, 1988Crossref, Medline, Google Scholar
33. Clarke CE, Shand D, Yuill GM, et al: Clinical spectrum of neuroleptic malignant syndrome (ltr). Lancet 2:969-970, 1988Crossref, Medline, Google Scholar
34. McCarthy A, Bourke S, Fahy J, et al: Fatal recurrence of neuroleptic malignant syndrome. British Journal of Psychiatry 152:558-559, 1988Crossref, Medline, Google Scholar
35. Levinson DF, Simpson GM: Neuroleptic-induced extrapyramidal symptoms with fever: heterogeneity of the "neuroleptic malignant syndrome." Archives of General Psychiatry 43:839-848, 1986Crossref, Medline, Google Scholar
36. Mann SC, Caroff SN, Bleier HR, et al: Lethal catatonia. American Journal of Psychiatry 143:1374-1381, 1986Link, Google Scholar
37. Castillo E, Rubin RT, Holsboer-Trachsler E: Clinical differentiation between lethal catatonia and neuroleptic malignant syndrome. American Journal of Psychiatry 146:324-328, 1989Link, Google Scholar
38. Levenson JL: Clinical differentiation between lethal catatonia and neuroleptic malignant syndrome (ltr). American Journal of Psychiatry 146:1241, 1989Link, Google Scholar
39. Shalev A, Hermesh H, Munitz H: The role of external heat load in triggering the neuroleptic malignant syndrome. American Journal of Psychiatry 145:110-111, 1988Link, Google Scholar
40. Stoudemire A, Luther JS: Neuroleptic malignant syndrome and neuroleptic-induced catatonia: differential diagnosis and treatment. International Journal of Psychiatry in Medicine 14:57-63, 1984Crossref, Medline, Google Scholar
41. Van Agtamael MA, van Harten PN: Malignant neuroleptic syndrome: complete anticoagulant treatment or not? Nederlands Tijdschrift voor Geneeskunde 136:1870-1872, 1992Google Scholar
42. Johnson SB, Alvarez WA, Freinhar JP: Rhabdomyolysis in retrospect: are psychiatric patients predisposed to this little-known syndrome? International Journal of Psychiatry in Medicine 17:163-171, 1987Google Scholar
43. Lee S, Merriam A, Kim TS, et al: Cerebellar degeneration in neuroleptic malignant syndrome: neuropathologic findings and review of the literature concerning heat-related nervous system injury. Journal of Neurology, Neurosurgery, and Psychiatry 52:387-391, 1989Crossref, Medline, Google Scholar
44. Rothke S, Bush D: Neuropsychological sequelae of neuroleptic malignant syndrome. Biological Psychiatry 21:838-841, 1986Crossref, Medline, Google Scholar
45. Konikoff F, Kuritzky A, Jerushalmi Y, et al: Neuroleptic malignant syndrome induced by single injection of haloperidol (ltr). British Medical Journal 289:1228-1229, 1984Crossref, Medline, Google Scholar
46. Legras A, Hurel D, Dabrowski G, et al: Protracted neuroleptic malignant syndrome complicating long-acting neuroleptic administration. American Journal of Medicine 85:875-878, 1988Crossref, Medline, Google Scholar
47. Rosenberg MR, Green M: Neuroleptic malignant syndrome: review of response to therapy. Archives of Internal Medicine 149:1927-1931, 1989Crossref, Medline, Google Scholar
48. Rosebush P, Stewart T: A prospective analysis of 24 episodes of neuroleptic malignant syndrome. American Journal of Psychiatry 146:717-725, 1989Link, Google Scholar
49. Sakkas P, Davis JM, Hua J, et al: Pharmacotherapy of neuroleptic malignant syndrome. Psychiatric Annals 21:157-164, 1991Crossref, Google Scholar
50. Addonizio G, Susman VL: ECT as a treatment alternative for patients with symptoms of neuroleptic malignant syndrome. Journal of Clinical Psychiatry 48:102-105, 1987Medline, Google Scholar
51. Scheftner WA, Shulman RB: Treatment choice in neuroleptic malignant syndrome. Convulsive Therapy 8:267-279, 1992Medline, Google Scholar
52. Davis JM, Janicak PG, Sakkas P, et al: Electroconvulsive therapy in the treatment of the neuroleptic malignant syndrome. Convulsive Therapy 7:111-120, 1991Medline, Google Scholar
53. Gold R, Lenox RH: Is there a rationale for iron supplementation in the treatment of akathisia? A review of the evidence. Journal of Clinical Psychiatry 56:476-483, 1995Medline, Google Scholar
54. Pelonero AL, Levenson JL, Silverman JJ: Neuroleptic therapy following neuroleptic malignant syndrome. Psychosomatics 26:946-948, 1985Crossref, Medline, Google Scholar
55. Wells AJ, Sommi RW, Crismon ML: Neuroleptic rechallenge after neuroleptic malignant syndrome: case report and literature review. Drug Intelligence and Clinical Pharmacy 22:475-480, 1988Crossref, Medline, Google Scholar
56. Susman VL, Addonizio G: Recurrence of neuroleptic malignant syndrome. Journal of Nervous and Mental Disease 176:234-241, 1988Crossref, Medline, Google Scholar
57. Rosebush PI, Stewart TD, Gelenberg AJ: Twenty neuroleptic rechallenges after neuroleptic malignant syndrome in 15 patients. Journal of Clinical Psychiatry 50:295-298, 1989Medline, Google Scholar
58. Levenson JL, Fisher JG: Long-term outcome after neuroleptic malignant syndrome. Journal of Clinical Psychiatry 49:154-156, 1988Medline, Google Scholar
59. Pope HG, Aizley HG, Keck PE, et al: Neuroleptic malignant syndrome: long-term follow-up of 20 cases. Journal of Clinical Psychiatry 52:208-212, 1991Medline, Google Scholar
60. Gelenberg AJ, Bellinghausen B, Wojcik JD, et al: Patients with neuroleptic malignant syndrome histories: what happens when they are rehospitalized? Journal of Clinical Psychiatry 50:178-180, 1989Google Scholar
61. Fernando MLD, Manchanda R, Kirk C: Neuroleptic malignant syndrome: a preventative program. Journal of Psychiatry and Neuroscience 17:31-33, 1992Medline, Google Scholar
62. Creese I, Hamblin MW, Leff SE, et al: CNS dopamine receptors, in Handbook of Psychopharmacology, Vol 17. Edited by Iversen LL, Iversen SD, Snyder SH. New York, Plenum, 1983Google Scholar
63. Velamoor VR, Norman RM, Caroff SN, et al: Progression of symptoms in neuroleptic malignant syndrome. Journal of Nervous and Mental Disease 182:168-173, 1994Crossref, Medline, Google Scholar
64. Caroff S, Rosenberg H, Gerber JC: Neuroleptic malignant syndrome and malignant hyperthermia (ltr). Lancet 1:244, 1983Crossref, Medline, Google Scholar
65. Downey GP, Caroff S, Beck S, et al: Neuroleptic malignant syndrome patient with unique ethical and physiologic features. American Journal of Medicine 77:338-340, 1984Crossref, Medline, Google Scholar
66. Tollefson G: A case of neuroleptic malignant syndrome: in vitro muscle comparison with malignant hyperthermia. Journal of Clinical Psychopharmacology 2:266-270, 1982Medline, Google Scholar
67. Scarlett JD, Zimmerman R, Berkovic SF: Neuroleptic malignant syndrome. Australian and New Zealand Journal of Medicine 13:70-73, 1983Crossref, Medline, Google Scholar
68. Bond WS: Detection and management of the neuroleptic malignant syndrome. Clinical Pharmacology 3:302-307, 1984Medline, Google Scholar
69. Blair DT, Dauner A: Neuroleptic malignant syndrome: liability in nursing practice. Journal of Psychosocial Nursing and Mental Health Services 31:5-12, 1993Medline, Google Scholar
70. Lannas PA, Packar JV: A fatal case of neuroleptic malignant syndrome. Medicine, Science, and the Law 33:86-88, 1993Crossref, Medline, Google Scholar
71. Lazarus A: Should neuroleptic malignant syndrome be treated in a private psychiatric hospital or a general hospital? General Hospital Psychiatry 12:245-247, 1990Google Scholar



