Research Risk for Persons With Psychiatric Disorders: A Decisional Framework to Meet the Ethical Challenge
Federal agencies historically and currently have expressed concern that adults who have psychiatric disorders may be a "vulnerable population" requiring special consideration and protections as research participants ( 1 , 2 , 3 , 4 , 5 ). In the late 1970s proposed federal regulations for the "institutionalized mentally infirm" recommended special protections and restrictions when involving this population as research participants ( 1 ). The proposed regulations were decried as conceptually unsound, increasing stigma and potentially undercutting research. Although these proposed regulations were never enacted, the impetus to establish federal regulations has persisted, and various presidential commissions ( 2 , 3 , 4 ) returned to recommending that "vulnerable individuals with psychiatric disorders" be afforded special protections in the research setting.
Currently, a commission formed by the Office for Human Research Protections of the Department of Health and Human Services (DHHS) is again considering the need for special regulations, and it has requested from the research community "information and comments about whether guidance or additional regulations are needed" for research with people who have psychiatric disorders ( 5 ).
Although no special DHHS regulations govern research with persons who have psychiatric disorders, institutional review boards (IRBs) typically consider studies involving such persons using a different standard than they use for studies involving adults who do not have psychiatric disorders. Factors involved in IRB decisions include degree of risk, prospect for direct benefit (a category usually reserved for treatment studies), and the extent to which researchers are able to take steps to mitigate risk ( 6 ). However, there is a lack of consensus on how to evaluate studies in which considerations of both degree of risk and vulnerability need to be made. There is considerable empirical evidence (including surveys of IRB administrators and reports of "real world" studies) documenting a wide range of variability in how IRBs evaluate risk ( 7 , 8 , 9 , 10 ). Achieving some reliability in risk assessment will become especially important if regulations are adopted that allow IRBs to restrict studies that are judged to pose greater than minor increment over minimal risk with no prospect for direct benefit ( 2 ). If such regulations were enacted, erroneous IRB judgments of risk might lead to the unnecessary prohibition of studies that pose little actual possibility of harm, or IRBs might require protective procedures that place an unnecessary burden on researchers ( 11 ).
Despite the lack of consensus described above, a body of research has emerged providing evidence that can help to inform the IRB decision-making process with regard to research with persons who have psychiatric disorders ( 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ). We reviewed this research in order to provide an evidence-based analysis of the concept of vulnerability, objective degree of risk, and procedures to mitigate risk in studies with persons in this population.
In this article, we summarize the literature and then offer a practical framework for evaluating the potential risk of studies and determining whether a study should be regarded to pose minimal risk, minor increment over minimal risk, or greater than minor increment over minimal risk. The purpose of the review and framework is to help researchers and IRBs to evaluate psychiatric research with more consistency and in a manner that is grounded in the existing evidence base.
Methods
To find relevant articles, searches of the MEDLINE (1966–2006), PsycINFO (1967–2006), and Google Scholar databases were conducted with combinations of the following terms: mental illness, vulnerable, psychiatric, schizophrenia, and depression combined with terms such as research risk, vulnerability, research harm, capacity, risk, and mitigation of risk. Additional searches used terms from identified articles, and we checked reference lists of these articles to locate other articles. Articles were included in the review if they addressed a relevant aspect of the material covered under the scope of the review.
Results
Vulnerability
Definition of vulnerability. The concept of vulnerability in research is rarely explicitly defined and has been criticized as being too broad to be useful ( 26 ). Some have misunderstood it as referring to vulnerability to potential harm through participation in research; however, it typically refers to vulnerability to undue coercion ( 2 , 27 ). In this respect, individuals with psychiatric disorders have been considered to be potentially vulnerable in two ways: because of their impaired capacity to provide informed consent ( 26 ) (capacity-based vulnerability) and because of their susceptibility to coercion as a result of the power differential between the investigator and the potential participant ( 1 , 28 ) (power-based vulnerability).
Although no formal regulations govern IRBs in the area of vulnerability to coercion in research with persons who have psychiatric disorders, IRBs are strongly urged to pay greater attention to evaluating whether participants have the ability to provide informed consent and to determine what additional safeguards need to be put into place to ensure that participants adequately understand the research procedures, risks, and benefits ( 2 , 3 , 4 ). However, the National Bioethics Advisory Commission (NBAC) report made it clear that although people with psychiatric disorders are considered to be a vulnerable population, not all persons with psychiatric disorders should be considered vulnerable ( 2 ).
Thus there is believed to be a range of capacity-based vulnerability among persons who have psychiatric disorders. Below we review the evidence for ranges in capacity-based vulnerability in this population and evidence that specific subgroups show a greater degree of impaired capacity than others.
Capacity-based vulnerability. Several studies have documented the degree to which persons with psychiatric disorders (particularly schizophrenia) are able to comprehend informed consent. Dunn's ( 24 ) review of research with persons with schizophrenia noted that two major conclusions can be drawn from these studies: that the majority of this population is able to understand the consent process and that on average these individuals show poorer understanding of research than persons without psychiatric disorders. These findings suggest that although diminished capacity is indeed an important consideration in regard to persons who have psychiatric disorders, it nevertheless is not universal or even typical.
We further note that important differences have been observed in the proportion of persons who demonstrate capacity to provide consent, depending on the recruitment source for the study, in particular, whether the sample is inpatient or outpatient. For example, a study with long-term inpatients found that as many as 67% of persons with schizophrenia performed in an inadequate manner on tests of decisional impairment ( 22 ); in contrast, two other studies ( 20 , 21 ) found that only roughly 20%–30% of persons with schizophrenia who were drawn from predominantly outpatient samples showed evidence of decisional impairment.
In addition to the studies conducted with persons with schizophrenia, a small number of studies have been conducted with persons with depressive disorders ( 30 , 31 , 32 ). Appelbaum and colleagues ( 30 ) found that more than 90% of participants with major depression demonstrated full comprehension of consent. Similarly, Stiles and colleagues ( 31 ) found that participants with depression did not score significantly worse on measures of understanding than participants from the community in a control group but scored better than participants with schizophrenia. Another study of comprehension found that participants with major depression performed somewhat more poorly than community participants but better than participants with schizophrenia and that a majority (over 85%) demonstrated very good comprehension ( 32 ). These studies suggest that the capacity of persons with depressive disorders to consent to research is not markedly worse than that of persons in the general population. Although some have suggested that individuals with underlying suicidality may manifest incapacity as a willingness to accept untoward risk ( 33 ), no data exist to support this notion.
On the basis of available evidence, we recommend that IRBs recognize that although persons with psychiatric disorders are at risk as a population for vulnerability to coercion, vulnerability is likely to fluctuate with mental state. It is also evident that capacity-based vulnerability to coercion is greater for persons with schizophrenia than for those with major depression (evidence regarding persons with bipolar disorder and other psychotic disorders is lacking). We conclude that capacity-based vulnerability to coercion should be considered a potential state rather than a trait among people with psychiatric disorders and that one way to gauge the likelihood of vulnerability is by determining whether the sample is drawn from persons in the active phase of their disorder, such as when they are in need of hospitalization or crisis services, or in the stable phase, such as when they are in receipt of ongoing services in the community.
Power-based vulnerability. Vulnerability due to a power differential has not been systematically studied among persons with psychiatric disorders. However, a wealth of social psychology literature provides evidence that individuals are generally more susceptible to social influence and to complying with the commands of authority figures when they are placed in institutional settings where their autonomy is restricted ( 34 ). In addition to those who are involuntarily hospitalized, the most vulnerable individuals in this respect are those who reside in correctional settings ( 35 ). Another consideration with regard to power-based vulnerability is related to cultural characteristics that might lead individuals to be more likely to defer to authority figures. A review of ethical issues related to the involvement of persons from racial-ethnic minority groups in psychiatric research found no studies that directly addressed this issue, but the authors suggested that groups such as unacculturated Asian and Hispanic immigrants might be more vulnerable to "defer" to medical authority when asked to sign informed consent forms ( 36 ). It has also been suggested that the need for money to purchase drugs can be a coercive factor in research with individuals who have active substance abuse problems, although research has not explored this issue ( 37 ).
We conclude that power-based vulnerability can be assumed to vary and that IRBs can address this issue by considering the setting in which an individual with a psychiatric disorder is approached for research participation, as well as by considering the potential impact of acculturation and the presence of co-occurring active substance abuse.
Risk
Standard definition of risk. Risk is determined by considering both the magnitude of potential harm and the likelihood of potential harm. Although there has been some disagreement over whether research risk encompasses two or three categories, the current predominant opinion is that three risk levels should be considered: minimal risk, minor increment over minimal risk, and greater than minor increment over minimal risk ( 6 , 38 ). There is a belief that the degree of risk that certain types of studies pose for persons with psychiatric disorders may be different from the degree of risk posed to others ( 2 ). Below, we synthesize findings from a variety of studies in order to provide preliminary guidance on the types of studies that can be classified in each of the three major risk categories.
Minimal-risk research. The concept of minimal-risk research was originally drawn from the Common Rule ( 4 ) and defined as follows: "the probability and magnitude of harm and discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests." Although there have been disagreements regarding this definition, it has remained the standard by which IRBs evaluate potential risk. NBAC's discussion of the concept of minimal-risk research with persons who have psychiatric disorders implied that some studies might be reasonably judged to fall into this category, but the discussion alerted IRBs to the possibility that there might be "special vulnerability to harm and discomfort" in this population, such that procedures considered to be of minimal risk for persons in the general population might not be considered to be of minimal risk for persons with psychiatric disorders (for example, emotional reactivity might lead persons with psychiatric disorders to become upset by certain questions). There is evidence that IRBs have reacted to this advice by taking a conservative posture, as a matter of course giving studies involving persons with psychiatric disorders a full board review ( 39 ). Our review of existing research evidence, however, suggests that this stance may be too restrictive. Below we discuss two common categories of studies that might be considered to be of minimal potential risk for most persons with psychiatric disorders on the basis of available evidence.
Survey- and interview-based studies, which may be considered of minimal risk, are defined here as studies in which persons with psychiatric disorders are asked questions about symptoms, daily life activities, thoughts, feelings, and opinions, either orally or by way of paper-and-pencil questionnaires. Three general categories of studies have examined the risk posed by this type of research: those that retrospectively examined the adverse reactions of persons with psychiatric disorders who participated in such studies ( 12 , 14 ), those that examined the expected risks of participation as judged by psychiatrists ( 15 , 16 , 17 ), and those that examined the expected risks as judged by persons with psychiatric disorders ( 15 , 16 , 17 , 18 ).
Findings from the retrospective studies indicated that less than 10% of persons with psychiatric disorders who participated in interview-based research experienced more than minimal anxiety ( 12 , 14 ) and that even fewer reported that this anxiety reached "severe" levels. Comparable rates of reports by participants of greater than minimal anxiety have been found in behavioral studies of psychiatric symptoms in populations without psychiatric disorders ( 40 , 42 ). These findings suggest that adverse reactions are unlikely, that they occur among people with psychiatric disorders only slightly more often than in the general population, and that they reach severe levels very rarely (if ever). Studies of psychiatrists have found that they regard questionnaire-based studies as the least risky category of research for people with psychiatric disorders and that they consider these studies to involve less risk than persons in this population would encounter in daily life ( 16 , 17 ). Findings from the third category of studies have found similar evidence. Persons with schizophrenia viewed questionnaire-based research as less risky than daily life and as the least risky type of research overall; a low rating of risk was related to their potential willingness to participate in such studies ( 19 , 41 ).
Routine physical examinations may also be considered to be of minimal risk. Two categories of studies have examined the risk posed by this type of research: those that examined the expected risks of participation as judged by psychiatrists ( 15 , 16 ) and those that examined the expected risks as judged by persons with psychiatric disorders ( 41 ). The first category of studies found that psychiatrists consistently rated research involving routine physical examination procedures as among the least risky categories of research for people with psychiatric disorders and that they considered that the risk involved is less than the risk that persons in this population would encounter in daily life ( 15 , 16 ). Similarly, the second category of studies found that persons with schizophrenia viewed research involving physical examination procedures as less risky than daily life and among the least risky types of research ( 17 ).
On the basis of the existing evidence, we conclude that both the probability and the magnitude of risk posed by survey- and interview-based studies and by studies that involve routine physical examinations and blood draws are small when conducted with persons who have psychiatric disorders. This conclusion is consistent with the general consensus that such research poses minimal risk when conducted with persons in the general population.
Minor increment over minimal risk. Minor increment over minimal risk is a category that exists in the federal regulations for research with children and has not been officially adopted in any regulations pertaining to adults. However, the category is commonly applied to research with vulnerable adults. A work group commissioned by the New York State Department of Health defined this category as consisting of studies in which "the probability and magnitude of harm … are only slightly greater … than those ordinarily encountered during the performance of routine physical or psychological examinations or tests" ( 43 ). Thus this category may include types of research in which the risks of harm occur slightly more frequently or are slightly more serious than those in the studies described above as being of minimal risk. Below we discuss two common categories of studies that might be considered to involve minor increment over minimal risk for most persons with psychiatric disorders on the basis of available evidence.
Some procedures involving physical examinations or medication administration may involve a minor increment over minimal risk. Two categories of studies have examined the risk posed by this type of research: those that examined the expected risks of participation as judged by psychiatrists ( 15 , 16 ) and those that examined the expected risks as judged by persons with psychiatric disorders ( 17 , 41 ). The first category of studies found that psychiatrists consistently rated studies involving more invasive physical procedures, such as undergoing an MRI with sedation or taking a new experimental medication, as a moderately risky category of research for people with psychiatric disorders and that they considered that the risk involved is less than or equal to the daily risks encountered by persons in this population ( 15 , 16 ). The second category of studies found that persons with schizophrenia viewed research involving these physical procedures to be about as risky as daily life and moderately more risky than the procedures that we have characterized as minimal-risk research ( 17 , 41 ).
Behavioral research on provocative topics may also involve a minor increment over minimal risk. Despite the evidence previously presented regarding the minimal risk of survey- and interview-based research, there is evidence that studies of provocative topics may present a greater likelihood and a greater magnitude of adverse reaction ( 13 ). Studies that specifically focus on traumatic stress, such as childhood sexual abuse and adult trauma and victimization, may be of special concern, especially when conducted with individuals who have experienced trauma. Our review found a greater likelihood of intense distress in such studies; in particular, a study conducted with psychiatric inpatients found that 24% became very upset as a result of participation ( 13 ). Similarly, research has found that persons meeting criteria for psychiatric disorders such as posttraumatic stress disorder (PTSD) and major depressive disorder were more likely to report experiencing emotional reactions in response to a "lengthy and intrusive" interview that included questions about trauma and victimization ( 19 ). However, the participants also reported that they did not regret participating. The authors of a recent review similarly concurred that there is greater evidence for distress in trauma-focused studies than in other behavioral research with persons who have psychiatric disorders ( 14 ).
Concerns have also been raised about research on other types of "provocative topics," such as studies involving violent or sexually charged imagery or descriptions, which may pose greater emotional risks ( 44 ). Although no research has examined reactions to such studies among persons with psychiatric disorders, persons with schizophrenia rated the expected risk of research that involved viewing an "upsetting image" as riskier that other types of survey research ( 41 ).
On the basis of the existing evidence, we conclude that more invasive physical procedures, such as an MRI with sedation and a trial of a new medication, as well as interviews about sensitive topics, can be reasonably expected to pose a minor increment over minimal risk among persons with psychiatric disorders.
Greater than minor increment over minimal risk. No clear definition exists for evaluating greater than minor increment over minimal risk in federal regulations. However, one IRB offered a useful definition, characterizing such studies as those in which either the probability of risk is likely (harm occurs for greater than 30% of participants) or the magnitude of risk is severe, even given a low frequency of occurrence ( 45 ).
Examples of study types that may fall into this risk category are medication washout studies, placebo-controlled medication studies, and symptom challenge studies. Shamoo ( 46 ) has expressed strong concerns for the appropriateness of these procedures. Some mental health consumers have also expressed their hesitancy to participate in these types of studies. Consumers who responded to a survey about their reasons for not participating in research gave the following reasons: the obligation to switch medications, the length of time required for stabilization, and lack of knowledge about whether the provided drug was a placebo ( 47 ). In previous studies psychiatrists have consistently rated three types of studies as riskier than the daily risks encountered by persons with psychiatric disorders: those that involve more than a two-day medication washout, those in which patients receive a medication that causes symptoms, and those in which patients receive a placebo instead of a medication ( 15 , 16 ). Studies that included persons with schizophrenia indicated that this population has similar views of research involving these physical procedures, rating them as riskier than daily life and significantly riskier than the studies characterized above as involving minor increment over minimal risk ( 17 , 41 ).
Some data are available on the actual risks of these types of studies. Wyatt and colleagues ( 48 ) examined the long-term effects of participating in conditions that involved placebo among 127 individuals with schizophrenia who were involved in medication trials conducted by the National Institute of Mental Health. All individuals who had been placed on placebo were eventually treated with antipsychotic medication. This research indicated that although all individuals who had received the placebo eventually returned to a baseline level of symptoms, the duration of their recovery period was typically six weeks after the restoration of medication. Although the eventual stability of the participants suggests that placebo-controlled studies do not cause irreparable harm, the length of time required to return to baseline suggests that they experienced a significant loss of functionality for a substantial period. Thus placebo-controlled studies may cause a great delay in the return to stability among persons with schizophrenia and the delay should be considered a risk of such research.
With regard to washout studies, Jeste and colleagues ( 49 ) reviewed relapse rates of more than 3,000 persons with schizophrenia who were "withdrawn" from antipsychotic medication in research trials. By 24 months, the relapse rate for these individuals was more than twice the rate for persons "maintained" on antipsychotic medications (59% compared with less than 27%). On the basis of these findings, it was recommended that, whenever possible, medication studies should not use lengthy washout periods but instead taper individuals off of previous medications in order to transition to new medications and, whenever possible, conduct studies in an inpatient environment to protect against harm associated with relapse. Grunebaum and colleagues ( 50 ) found that short-term medication washout from antidepressants and mood stabilizers (roughly two weeks) posed few risks for persons with major depressive disorder while they were hospitalized. Risks of relapse may be less of a concern for short-term washout from antidepressants.
As previously stated, symptom challenge studies have been heavily criticized by Shamoo ( 46 ), who reviewed studies involving the use of amphetamines and other chemicals to trigger symptoms among persons with PTSD and schizophrenia, stating that such studies compromise the psychiatric stability of those participating in them. However, he provided little empirical data in his discussion of the effects of these studies. Lahti and colleagues ( 51 ), however, reviewed the long-term outcome of 25 persons with schizophrenia who participated in ketamine challenge studies and found no support for serious adverse effects over the course of eight months. Carpenter ( 52 ) also reviewed several ketamine challenge studies and found that only three patients terminated participation in the research because of discomfort (two from the study group and one from the placebo group). He concluded that there was no evidence that ketamine induction led to a relapse. Data from studies of patients with disorders other than schizophrenia are not available. Furthermore, few data are available on the extent to which other challenge studies might create risk of relapse.
On the basis of the available data, we conclude that studies that involve placebo control, lengthy medication withdrawal, and symptom challenge present risks that are potentially of greater frequency or severity than the studies described above as involving minor increment over minimal risk. Specifically, although the effects of these procedures may be mitigated in the long term, participants with some psychiatric disorders who participate in placebo studies and withdrawal studies may be likely to experience more severe symptoms for a longer period, which may have an impact on their functioning. Although challenge studies are controversial, no evidence of long-term effects has been found for the majority of participants, but some studies have suggested an increased risk in rare cases, which may constitute a greater than minor increment over minimal risk.
Mitigation of risk and vulnerability
In addition to concerns about vulnerability and risk, IRBs that review studies involving persons with psychiatric disorders consider the effort that researchers will take to mitigate risk of harm or vulnerability to coercion.
We have argued that potential vulnerability to coercion into research participation is a state—often associated with acute symptoms—that may be unalterable by researchers. However, efforts can be made to increase the accessibility of the informed consent procedures and effectively mitigate vulnerability to coercion. Some empirical evidence exists regarding the efficacy of repeated disclosure of information to enhance an individual's ability to provide informed consent. One study found that all participants were eventually able to correctly answer questions about the psychopharmacological clinical trials in which they were being asked to participate ( 23 ). Another study found that iterative feedback during informed consent discussions was associated with improvement in research comprehension scores among participants ( 31 ). Implementation of these procedures, particularly in studies with complicated research designs, may decrease the need for including third parties such as monitors.
A small body of literature has addressed ways to mitigate risk in research that is potentially upsetting ( 6 , 53 , 54 , 55 ). No articles are based on empirical studies; instead, they recommend approaches based on investigator experience and consensus. In addition, these articles have not specifically focused on research involving persons with psychiatric disorders but have considered ways to moderate upset among study participants more generally. Some common strategies recommended include closely monitoring respondent distress throughout the research process, allowing and encouraging participants to take the opportunity to use coping techniques to deal with distress, using clinical interventions derived from psychotherapy to moderate distress when it is encountered, and making a referral for crisis intervention or hospitalization when necessary. Although systematic research is clearly needed on the degree to which such interventions would mitigate the risk of research studies with persons who have psychiatric disorders, it stands to reason that experienced and well-trained research teams will be more likely to carry out these risk management strategies. For this reason, we recommend that IRBs consider the degree of clinical experience and training of a proposed study's research personnel when evaluating risk.
Discussion
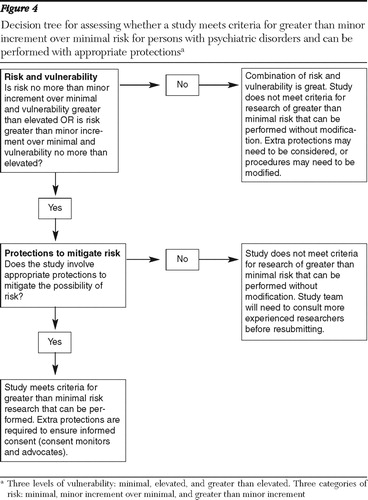
What use can IRBs make of the available evidence on vulnerability and risk of research with persons who have psychiatric disorders? To provide a structured response to this question, we present a framework designed to guide IRBs in their evaluation of such studies. The framework is represented in a series of diagrams and decision trees in Figures 1–4.
First, the evaluator considers the continua of risk and vulnerability illustrated in Figure 1 . Regarding vulnerability, the evaluator considers both power-based vulnerability (such as whether participants are institutionalized and in a position of diminished autonomy) and capacity-based vulnerability (which is related to participants' likely ability to understand consent). By considering both factors, the evaluator judges whether vulnerability is minimal, elevated, or greater than elevated. For example, a stable outpatient population might be regarded as having minimal vulnerability to coercion because they have minimal power-based and capacity-based vulnerability.

A similar approach guides judgments of risk. The evaluator judges the relative risk of the procedures on the basis of the evidence presented above (for example, if the study uses only interview or physical examination procedures, risk is minimal). In judging risk, the evaluator simultaneously considers participants' potential sensitivity to the study procedures, considering, for example, whether participants are likely to be highly symptomatic when they engage in the procedures. By considering both factors, the evaluator can judge whether the study poses minimal, elevated, or greater than elevated risk. For example, a study that uses questions about prior trauma might be judged to present elevated risk if it is implemented with a sample of persons who have PTSD.
The research evaluator then proceeds to Figures 2–4, which consist of a series of decision trees that consider three factors in evaluating the overall potential risk of the study: vulnerability, risk of study procedures, and protections to mitigate risk. Judgments of degree of risk and vulnerability are made after considering Figure 1 , while the issue of protections to mitigate risk is also considered by examining the experience of the research team and the protections they propose to implement.
The research evaluator begins with Figure 2 in order to consider whether a study qualifies as a minimal-risk study. As can be seen in Figure 2 , the framework allows a reviewer to judge a study as presenting minimal risk only if risk and vulnerability are both minimal and the study contains adequate protections to mitigate risk. If one of these conditions is not met, the evaluator is directed to Figure 3 in order to determine whether the study meets criteria for minor increment over minimal risk. A study can receive this designation if risk and vulnerability are no more than elevated but not if either exceeds this designation. Furthermore, studies need to demonstrate evidence of adequate protections in order to receive this designation.


If a study is judged as not meeting criteria for minor increment over minimal risk, the evaluator is directed to Figure 4 in order to determine whether the study meets criteria for a study with greater than minor increment over minimal risk that can be performed without modification. In this figure, the evaluator first considers whether the study involves procedures with greater risk (such as placebo control) and participants with no more than elevated vulnerability or highly vulnerable participants and procedures with no more than elevated risk. If these conditions are met, the evaluator then assesses whether appropriate protections are in place for this type of study. If these conditions are not met (that is, if the study involves procedures that present greater than minor increment over minimal risk and highly vulnerable participants), the evaluator then should consider whether greater protections are in place or whether the study should be conducted with less vulnerable persons (such as an outpatient sample). In appropriate circumstances, if protections are not offered or the scientific merit of the study is not clear, the IRB may decide that the study should not be conducted or that the design should be altered.
Conclusions
We conclude that although more research is needed on the risks of research participation among persons with psychiatric disorders, there is currently sufficient evidence that many common types of research present minimal risk or only a minor increment over minimal risk for large segments of this population, as they do for persons in the general population. Vulnerability must be evaluated as a range of two dimensions: capacity and voluntariness. For studies that recruit less vulnerable samples and involve only minimal-risk procedures, IRBs may categorize the research as minimal risk. Minimal-risk studies could be reviewed in an expedited manner as long as an individual who is familiar with research involving persons with psychiatric disorders is available on the IRB. Measures such as consent auditors are not warranted for such low-risk studies. For studies using procedures that we have characterized as posing minor increment over minimal risk, full board review is likely necessary, but IRBs need not call for consent monitors when it is determined that persons in less vulnerable states would be recruited for the research, although a formal assessment of understanding of consent may be required. In other cases in which procedures that we have characterized as greater than minor increment over minimal risk are proposed, it is probably appropriate for IRBs to recommend that consent monitors be used in order to guarantee that participants are not unwittingly recruited into research that presents greater potential for harm.
In sum, we believe that the approach we recommend, if adopted, will lead IRBs to conduct more consistent, reasonable, and appropriate reviews of proposed studies with persons who have psychiatric disorders.
Acknowledgments and disclosures
Preparation of the manuscript was supported by grant K23MH066973 from the National Institute of Mental Health (NIMH) to Dr. Yanos and by grant P20AA015630 from the National Institute on Alcohol Abuse and Alcoholism and grants R01MH062665-05 and R01MH61017 from NIMH to Dr. Stanley.
The authors report no competing interests.
1. Research Involving Those Institutionalized as Mentally Infirm. Washington, DC, Department of Health, Education and Welfare, 1978Google Scholar
2. Exhibit A: Research involving persons with psychiatric disorders that may affect decision making capacity, in National Bioethics Advisory Commission 1998–1999 Biennial Report. Washington, DC, National Bioethics Advisory Commission, 1999. Available at www.bioethics.gov Google Scholar
3. Research Involving Individuals With Questionable Capacity to Consent: Points to Consider. Washington, DC, Department of Health and Human Services, Office of Intramural Research, Mar 11, 1999. Available at www.grants.nih.gov/grants Google Scholar
4. Special classes of subjects, in Institutional Review Board Guidebook. Washington, DC, US Department of Health and Human Services, 2007. Available at www.hhs.gov/ohrp Google Scholar
5. Office for Human Research Protections, Department of Health and Human Services: Request for information and comments on research that involves adult individuals with impaired decision-making capacity. Federal Register 72:50966–50970, 2007Google Scholar
6. American Psychiatric Association's Task Force on Research Ethics: Ethical principles and practices for research involving human participants with mental illness. Psychiatric Services 57:552–557, 2006Google Scholar
7. McWilliams NA, Jones EL, Davies RJ: Problematic variation in local institutional review of a multicenter genetic epidemiology study. JAMA 290:360–366, 2003Google Scholar
8. Silverman H, Hull SC, Sugarman J: Variability among institutional review boards' decisions within the context of a multicenter trial. Critical Care Medicine 29:235–241, 2001Google Scholar
9. Larson E, Bratts T, Zwanziger J, et al: A survey of IRB process in 68 US hospitals. Journal of Nursing Scholarship 36:260–264, 2004Google Scholar
10. Hirshon JM, Krugman SD, Witting MD, et al: Variability in institutional review board assessment of minimal-risk research. Academic Emergency Medicine 9:1417–1420, 2002Google Scholar
11. Michels R: Are research ethics bad for our mental health? New England Journal of Medicine 340:1427–1430, 1999Google Scholar
12. Boothroyd RA: The impact of research participation on adults with severe mental illness. Mental Health Services Research 2:213–222, 2000Google Scholar
13. Newman E, Kaloupek DG: The risks and benefits of participating in trauma-focused research studies. Journal of Traumatic Stress 17:383–394, 2004Google Scholar
14. Jorm AF, Kelly CM, Morgan AJ: Participant distress in psychiatric research: a systematic review. Psychological Medicine 27:917–926, 2007Google Scholar
15. Roberts LW, Warner TD, Hammond KG, et al: Assessments by patients with schizophrenia and psychiatrists of relative risk of research procedures. Psychiatric Services 57:1629–1635, 2006Google Scholar
16. Roberts LW, Dunn LB, Hammond KG, et al: Do research procedures pose relatively greater risk for healthy persons than for persons with schizophrenia? Schizophrenia Bulletin 32:153–158, 2006Google Scholar
17. Roberts LW, Warner TD, Brody JL, et al: Patient and psychiatrist ratings of hypothetical schizophrenia research protocols: assessment of harm potential and factors influencing participation decisions. American Journal of Psychiatry 159:573–584, 2002Google Scholar
18. Stanley B, Stanley M, Lautin A, et al: Preliminary findings on psychiatric patients as research participants: a population at risk? American Journal of Psychiatry 138:669–671, 1981Google Scholar
19. Widom CS, Czaja S: Reactions to research participation in vulnerable subgroups. Accountability in Research 12:115–138, 2005Google Scholar
20. Carpenter WT, Gold JM, Lahti AC, et al: Decisional capacity for informed consent in schizophrenia research. Archives of General Psychiatry 5:533–538, 2000Google Scholar
21. Palmer BW, Dunn LB, Appelbaum PS, et al: Assessment of capacity to consent to research among older persons with schizophrenia, Alzheimer disease, diabetes mellitus. Archives of General Psychiatry 62:726–733, 2005Google Scholar
22. Kovnick JA, Appelbaum PS, Hoge SK, et al: Competence to consent to research among long-stay inpatients with chronic schizophrenia. Psychiatric Services 54:1247–1252, 2003Google Scholar
23. Wirshing DA, Wirshing WC, Marder SR, et al: Informed consent: assessment of comprehension. American Journal of Psychiatry 155:1508–1511, 1998Google Scholar
24. Dunn LB: Capacity to consent to research in schizophrenia: the expanding evidence base. Behavioral Sciences and the Law 24: 431–445, 2006Google Scholar
25. Rosenstein DL, Miller FG: Ethical considerations in psychopharmacological research involving decisionally impaired subjects. Psychopharmacology 171:92–97, 2003Google Scholar
26. Levine C, Faden R, Grady C, et al: The limitations of "vulnerability" as a protection for human research participants. American Journal of Bioethics 3:44–49, 2004Google Scholar
27. Vulnerable Populations. Durham, NC, Duke University, Office of Research Support, 2007. Available at www.ors.duke.edu/irb Google Scholar
28. Silvers A: Historical vulnerability and special scrutiny: precautions against discrimination in medical research. American Journal of Bioethics 3:56–57, 2004Google Scholar
29. Rosenfeld B, Turkheimer E, Gardner W: Decision making in a schizophrenic population. Law and Human Behavior 16:651–662, 1992Google Scholar
30. Appelbaum PS, Grisso T, Frank E, et al: Competence of depressed patients for consent to research. American Journal of Psychiatry 156:1380–1384, 1999Google Scholar
31. Stiles PG, Poythress NG, Hall A, et al: Improving understanding of research consent disclosures among persons with mental illness. Psychiatric Services 52:780–785, 2001Google Scholar
32. Cohen BJ, McGarvery EL, Pinkerton RC, et al: Willingness and competence of depressed and schizophrenic inpatients to consent to research. Journal of the American Academy of Psychiatry and the Law 32:134–143, 2004Google Scholar
33. Fisher CB, Pearson JL, Kim S, et al: Ethical issues in including suicidal individuals in clinical research. IRB: Ethics and Human Research 24:9–14, 2002Google Scholar
34. Zimbardo PG, Leippe MR.: The Psychology of Attitude Change and Social Influence. New York, McGraw-Hill, 1991Google Scholar
35. Peternelj-Taylor AC: Conceptualizing nursing research with offenders: another look at vulnerability. International Journal of Law and Psychiatry 28:348–359, 2005Google Scholar
36. Miskimen T, Marin H, Escobar J: Psychopharmacological research ethics: special issues affecting US ethnic minorities. Psychopharmacology 171:98–104, 2003Google Scholar
37. Fisher CB: Ethics in drug abuse and related HIV risk research. Applied Developmental Science 8:91–103, 2004Google Scholar
38. Wendler D, Prasad K: Core safeguards for clinical research with adults who are unable to consent. Annals of Internal Medicine 135:514–523, 2001Google Scholar
39. Submission Guidelines for All New Studies. Kokomo, Indiana University Kokomo, Institutional Review Board for the Protection of Human Subjects. Available at www.iuk.edu/∼koirb/submissionguidelines.shtml Google Scholar
40. Boothroyd R, Best K: Emotional reactions to research participation and the relationship to understanding of informed consent disclosure. Social Work Research 27:242–251, 2003Google Scholar
41. Roberts LW, Hammond KG, Hoop J: An inverse relationship between perceived harm and participation willingness in schizophrenia research protocols. American Journal of Psychiatry 163:2002–2004, 2006Google Scholar
42. Jacomb PA, Jorm AF, Rodgers B, et al: Emotional response of participants to a mental health survey. Social Psychiatry and Psychiatric Epidemiology 34:80–84, 1999Google Scholar
43. Recommendations on the Oversight of Research Involving the Protected Classes. Albany, New York State Department of Health, Advisory Work Group on Human Subject Research Involving the Protected Classes, 1998. Available at purl.org/net/nysl/nysdocs/49377072 Google Scholar
44. Labott SM, Johnson TP: Psychological and social risks of behavioral research. IRB: Ethics and Human Research 26:11–15, 2004Google Scholar
45. Assessing the Research Risk. St Louis, Washington University, Human Research Protection Office, 2004. Available at https://hrpo.wustl.edu/HRPO/resource/doc/DO000748/minimalriskguideline.rtf Google Scholar
46. The Unethical Use of Human Beings in High Risk Research Experiments. Testimony by Adil E Shamoo before the Subcommittee on Oversight, Committee on Veterans Affairs, US House of Representatives, Apr 1, 1999. Available at www.house.gov/va/hearings/schedule106/apr99/4-21-99J/shamoo.htm Google Scholar
47. Hall LL: NAMI Consumer Views of Research. Arlington, Va, National Alliance on Mental Illness, Jan 2001. Available at www.nami.org Google Scholar
48. Wyatt RJ, Henter ID, Bartko JJ: The long-term effects of placebo in patients with chronic schizophrenia. Biological Psychiatry 46:1092–1105, 1999Google Scholar
49. Jeste DV, Palmer BW, Harris MJ: Neuroleptic discontinuation in clinical and research settings: scientific issues and ethical dilemmas. Biological Psychiatry 46:1050–1059, 1999Google Scholar
50. Grunebaum MF, Oquendo MA, Burke AK, et al: Clinical impact of a 2-week psychotropic medication washout in unipolar depressed inpatients. Journal of Affective Disorders 75:291–296, 2003Google Scholar
51. Lahti AC, Warfel D, Michaelidis T, et al: Long-term outcome of patients who receive ketamine during research. Biological Psychiatry 49:869–875, 2001Google Scholar
52. Carpenter WT: The schizophrenia ketamine challenge study debate. Biological Psychiatry 46:1081–1091, 1999Google Scholar
53. Koocher GP: Using the CABLES model to assess and minimize risk in research: control group hazards. Ethics and Behavior 12:75–86, 2002Google Scholar
54. Kavanaugh K, Ayres L: "Not as bad as it could have been": assessing and mitigating harm during research interviews on sensitive topics. Research in Nursing and Health 21:91–97, 1998Google Scholar
55. Oquendo MA, Stanley B, Ellis SP, et al: Protection of human subjects in intervention research for suicidal behavior. American Journal of Psychiatry 161:1558–1563, 2004Google Scholar



