Agreement Between Patients With Schizophrenia and Providers on Factors of Antipsychotic Medication Adherence
Schizophrenia occurs in 1 percent of the population, but patients with schizophrenia occupy more than 40 percent of psychiatric hospital beds ( 1 ). The annual U.S. cost of schizophrenia is estimated at $33-$65 billion, making it one of the most expensive psychiatric disorders in terms of direct treatment costs and indirect costs of lost productivity and expenditures for public assistance ( 2 , 3 ). Although antipsychotic medications are an essential component for treating patients with schizophrenia, adherence continues to be a major problem ( 4 , 5 ). Nonadherence to antipsychotic medication treatment leads to several important clinical and economic problems, including psychotic relapse, increased clinic and emergency department visits, and rehospitalization ( 2 , 6 , 7 , 8 , 9 ). In a comprehensive literature review of neuroleptic withdrawal, the mean cumulative relapse rate was 53 percent for patients withdrawn from antipsychotic medication and 16 percent for those whose illness was managed with antipsychotic medication ( 10 ). In addition to these costly clinical problems, there is growing evidence that nonadherence to antipsychotic medications substantially impairs health-related quality of life and functioning ( 11 , 12 , 13 ).
Efficacious antipsychotic regimens frequently do not achieve their potential because of nonadherence. In a review of the antipsychotic medication adherence literature, Cramer and Rosenheck ( 4 ) reported a median nonadherence rate of 42 percent. In a literature review using stricter inclusion criteria, Lacro and colleagues ( 5 ) reported a median nonadherence rate of 47 percent. They found that the most consistently reported and potentially modifiable risk factors for nonadherence were patient-related factors (poor insight into having a mental illness, negative attitude or subjective response toward antipsychotic medication, and substance abuse) and environment-related factors (poor therapeutic alliance) ( 5 ). These findings are consistent with findings from the recent Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in which 75 percent of patients stopped phase I antipsychotic medication within 18 months. The most common reason for stopping was simply the patient's decision to discontinue, not lack of efficacy or side effects ( 14 ).
Investigators have tested a number of interventions to improve antipsychotic medication nonadherence. In their recent review, Zygmunt and colleagues ( 15 ) identified five intervention categories—individual, family, group, community, and mixed—and found that the individual interventions tended to be the most successful. Successful individual interventions used behavioral tailoring ( 16 ) and motivational interviewing ( 17 ). However, a subsequent motivational interviewing intervention showed no advantage over nonspecific counseling ( 18 ). A successful mixed intervention used in the Veterans Affairs (VA) health care system used a behavioral tailoring component ( 19 ).
Two important observations emerge from review of successful medication adherence interventions. First, each of the successful interventions had a strong patient-centered focus. This feature is not surprising, given that the successful intervention strategies—behavioral tailoring and motivational interviewing—are by definition centered on the patient. Second, none of these interventions have been widely adopted, probably because of the significant costs associated with implementation.
In response to the continuing challenge of antipsychotic medication adherence, we began the process of developing a sustainable patient-centered intervention using the intervention mapping process ( 20 , 21 ). The first step in the intervention mapping process is to assess needs and capacities, which includes a literature review to define the problem, "going to the source" by asking patients and their providers about medication adherence, reviewing available adherence models and theories, and organizing needs and capacities by importance and modifiability. Subsequent steps in the intervention mapping process include defining objectives and the changes necessary to meet those objectives, reviewing available interventions and strategies to address the problem, producing and refining the intervention, implementing it, and evaluating it.
In "going to the source," we asked patients and their mental health providers to describe their explanatory models of schizophrenia and the barriers, facilitators, and motivators associated with antipsychotic medication adherence. Previous qualitative studies of medication adherence among patients with schizophrenia focused on medication knowledge ( 22 ) and medication side effects ( 23 ). Previously used models of medication adherence in this population were largely derived from the general medical literature and clinical experience, not patient self-report ( 24 , 25 , 26 ). To our knowledge, general models, such as the health belief model ( 27 ) and the health decision model ( 28 ), have not been informed by data from patients with schizophrenia.
In this article we describe and compare patient and provider explanatory models for schizophrenia and perceived barriers, facilitators, and motivators of antipsychotic medication adherence. These data will provide the building blocks for constructing a sustainable patient-centered intervention to improve antipsychotic medication adherence.
Methods
We recruited VA and non-VA outpatients diagnosed as having schizophrenia or schizoaffective disorders. We asked mental health providers to refer patients who were either medication adherent or nonadherent for an in-depth audiotaped interview about their experience with schizophrenia. However, because only patients who were currently taking their medication were referred, we asked all patients to discuss periods when they stopped and when they resumed taking their antipsychotic medication. Additional inclusion criteria included a clinical diagnosis of schizophrenia or schizoaffective disorder, prescribed oral antipsychotic medication other than clozapine for more than six months, age 20-70 years, score below 10 on the Blessed Orientation-Memory-Concentration Test ( 29 ), and access to a telephone. We excluded patients on clozapine because of the adherence criteria required to start clozapine and the more frequent monitoring required for clozapine. Additional exclusion criteria included a current clinical diagnosis of a substance use disorder or a serious physical health problem, such as seizure disorder or unstable angina. Patients with a substance use disorder were excluded because they are typically referred to programs that specialize in dual diagnosis and our goal was to develop an intervention for general mental health or intensive case management settings. Institutional review board approval was obtained from the University of Arkansas for Medical Sciences, and written informed consent was obtained from patient and provider participants.
Twenty-six patients (15 VA and 11 non-VA) completed in-depth qualitative interviews. Patients were recruited from either a standard outpatient setting or an intensive case management setting. Recruitment and interviewing of the VA sample were completed first. Data saturation determined the number of patients in the VA and non-VA subsamples. Recruitment ended when no new data were obtained from three consecutive interviews in each subsample. Mean± SD patient age was 44.3±9.9 years. Of the sample, 23 patients (89 percent) were male, 15 patients (58 percent) were Caucasian, 11 (42 percent) were African American, 11 (42 percent) completed some college, nine (35 percent) were working full- or part-time, and six (23 percent) lived in a supervised setting.
Seventeen mental health providers participated in the study (six were the provider for more than one patient). Eight were prescribing providers (four doctors of medicine, three prescribing nurses, and one doctor of pharmacy), and nine were nonprescribing providers (four social workers, three nurses, and two other providers). The inclusion criterion for providers was provision of direct care to a participating patient for at least six months. The median time in which providers had been involved in the care of each patient was 36 months (range of six to 144 months). The median number of patient visits to the participating provider per year was 19 (range of two to 260 visits). Thirteen patients (50 percent) received a home visit from the participating provider, and 20 patients (77 percent) received case management services.
Questions from an interview guide about explanations of illness and factors affecting medication adherence
Opening question
What is it like to live with the problem you are treated for in the mental health clinic on a daily basis?
Explanatory model probe questions
What do you call the mental illness you have?
What do you think caused this illness?
What are the main problems this illness has caused for you?
How do you know if this illness is getting worse?
What causes this illness to get worse?
What do you do to keep this illness from getting worse?
What does being healthy mean to you?
What do you do to stay healthy?
Barrier, facilitator, and motivator probe questions
What kinds of things make it harder for you to take your medication?
What kinds of things make it easier for you to take your medication?
What kinds of things make you want to take your medication?
Patient data collection
The research team developed a semistructured interview guide to facilitate and focus patient data collection. The interview guide started with an open-ended question and included more specific probe questions (see box, this page). Depth and richness were achieved by folding the probe questions and additional clarifying questions smoothly into the interview. We used a focused ethnographic approach; that is, we defined the topics of the semistructured interview guide a priori and attempted to understand the patients' responses in depth.
Explanatory models are individually constructed in response to a particular illness. They cover the perceived causes and significance of the illness, as well as the expected outcomes of treatment ( 30 ). Because they often form the basis of clinical communication, explanatory models can affect treatment adherence and clinical outcomes ( 31 ). We asked eight questions about explanatory models for schizophrenia, including seven covering the illness domains defined by Kleinman ( 30 ): name, cause, problems, recognition of increasing symptoms, causes of increasing symptoms, recognition of health, and maintenance of health. We asked patients to describe their own explanatory models for schizophrenia and providers to describe the explanatory model for their patient. Similarly, we asked patients to identify their medication adherence barriers, facilitators, and motivators, and we asked providers to identify the patient's barriers, facilitators, and motivators.
Two research assistants conducted the qualitative interviews with patients, one with a master's degree and one with a bachelor's degree. Both were trained in qualitative interviewing before conducting patient interviews. Training included videotaped instruction, assigned readings, observation of live interviews, and observation by trainers while conducting practice interviews. The patients' qualitative interviews were conducted in person, audiotaped, and held at a location determined by the patient (usually in the clinic or at the patient's residence). Initial interviews took 30 to 60 minutes. Audiotapes were transcribed verbatim within one week of the interview. Initial analysis of the first interview was limited to checking for patient responses that were not clear and for unasked probe questions. Such issues were covered in a follow-up interview conducted in person or over the telephone within two weeks of the initial interview. Follow-up interviews took ten to 15 minutes. All patient interviews and field notes were audiotaped, transcribed verbatim, checked for accuracy, and entered into the Ethnograph software program ( 32 ), which assigns line numbers to interview transcripts and facilitates the coding, organization, and analysis of narrative data.
Provider data collection
A mental health provider for each patient completed an open-ended paper-and-pencil questionnaire, keeping in mind the patient who completed the qualitative interview. The first part of the questionnaire asked about the explanatory model for mental illness experienced by the patient, and the second part asked about the patient's medication adherence barriers, facilitators, and motivators. Provider responses, which varied from a single word to several sentences, were also entered into the Ethnograph program.
Data analysis
In keeping with the patient-centered approach of this project, only the patient data were used to identify barriers, facilitators, and motivators. We used an inductive approach to the analysis, starting with the raw data (transcripts), and followed an iterative process of content analysis and constant comparison to identify patterns and commonalities within and across patient responses. The result was an organization of the data from the specific (transcript data) to the abstract (barrier, facilitator, and motivator domains). [A data flow diagram is available as an online supplement to this article at ps.psychiatryonline.org.]
Research personnel were trained in qualitative data analysis with transcripts from other studies and then jointly coded the first interview for this study. Subsequent interviews were coded independently by at least two research personnel and then discussed by the team to arrive at consensus. If coders initially disagreed on how to code a section of the transcript, each coder discussed his or her coding rationale until they reached consensus about which coding best fit the data. Consensus was achieved in all cases. The code book evolved over time as additional categories were identified. When new categories emerged, previously coded interviews were recoded to include the new categories. The research team recorded all coding and thematic decisions to provide an audit trail of the analytic process ( 33 ).
Initial interrater agreement on assigning barrier, facilitator, and motivator codes to the patient transcripts was 84 percent (226 of 268 cases; interrater agreement was conducted on approximately half of the patient transcripts). Interrater agreement on whether the patient and provider agreed on explanatory model domains was 89 percent (158 of 177). Interrater agreement on whether the patient and provider agreed on medication adherence barriers, facilitators, and motivators was 90 percent (69 of 77).
Results
Explanatory model
Patient and provider factors within each of the seven explanatory model domain are summarized in Table 1 . It is striking that patients and their providers emphasized different factors within each explanatory model domain except name of illness. For example, a much greater proportion of patients than of providers cited stress as the cause of their illness or as a factor that worsens it. Patients also were more likely to identify improved functioning and less likely to identify decreased symptoms as signs of health than were providers. Patients were less likely than providers to identify medication adherence as an important factor in controlling symptoms or maintaining health. In addition, patients were more likely than providers to identify stigma as a problem and to identify stress reduction and engaging in spiritual activity as important to maintaining health.
 |
a The number of factors may not add up to 26 (or 100 percent) because of multiple responses or lack of response by patient or provider.
Overall explanatory model agreement between patients and their providers was 66 percent (117 of 177). Agreement varied from 40 to 100 percent among the seven explanatory model domains ( Table 2 ). Examples of patient and provider quotes demonstrating agreement and disagreement for each of the explanatory model domains appear in Table 2 . The domains with the lowest agreement were cause of illness and signs of health. Most patients cited stress as the cause of their illness, whereas providers cited either biological or unknown causes. In addition, most patients cited improved functioning as a sign of health, whereas most providers cited decreased symptoms.
 |
Within the cause-of-illness domain, 44 percent (11 of 25) of providers who gave an answer responded that they did not know the cause compared with 8 percent (two of 25) of patients. In both cases where patients responded that they did not know the cause of their illness, so did their providers. Within the signs-of-health domain, one provider reported not knowing what being healthy would mean to his or her patient and the patient responded the same. Among all participants who responded to a given domain, 3 percent (6 of 179) of patients and 10 percent (18 of 177) of providers said "I do not know."
Barriers, facilitators, and motivators
We initially coded 477 patient-identified barriers, facilitators, and motivators for medication adherence: 174 barriers, 127 facilitators, and 176 motivators. Redundant codes were consolidated, and codes were organized into eight mutually exclusive categories: environment, side effects, relationship between provider and family, insight and knowledge, symptoms and outcomes, substance abuse, stigma, and dosing. All eight categories were represented within the barrier domain. Four categories—environment, provider-family relationship, insight and knowledge, and dosing—were represented in the facilitator domain, and three categories—environment, symptoms and outcomes, and provider-family relationship—were represented in the motivator domain. The large decrease from unique items to categories in the motivator domain was due to overlap; 88 percent (64 of 73) of the unique motivator items were in the symptoms and outcomes category.
Tables 3 , 4 , and 5 provide examples of the barrier, facilitator, and motivator data analysis process. We list factors within each category, the characteristics of each factor, and sample quotes (raw data) for selected characteristics. [Expanded versions of the tables are available as an online supplement to this article at ps.psychi atryonline.org.] Patients also identified potential "remedies"—that is, facilitators and motivators—for many of the identified barriers. There were, however, three barrier categories for which patients did not provide potential remedies: side effects, stigma, and substance abuse. There were also two categories, environment and provider-family relationship, in which patients identified factors in each of the barrier, facilitator, and motivator domains.
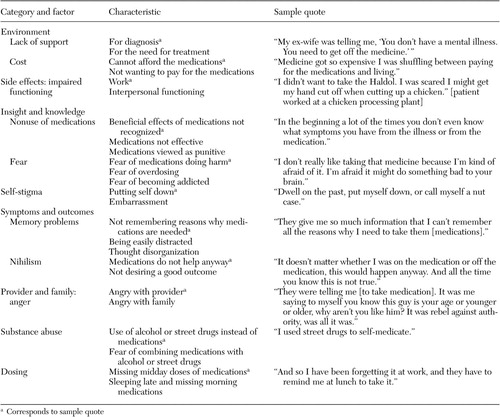 |
 |
 |
The overall agreement on barriers, facilitators, and motivators between patients and their providers was 61 percent (47 of 77): 54 percent (14 of 26) for barriers, 64 percent (16 of 25) for facilitators, and 65 percent (17 of 26) for motivators. All patients identified at least one barrier, and 85 percent (22 of 26) of their providers were able to identify at least one patient barrier. Most patients (24 of 25, or 96 percent) and 88 percent (22 of 25) of providers were able to identify at least one patient facilitator, and all patients and 88 percent (23 of 26) of providers were able to identify at least one patient motivator.
We also examined the relationship between the level of explanatory model agreement and barrier, facilitator, and motivator agreement. Low explanatory model agreement was defined as no agreement or agreement on one domain of seven, and high agreement was defined as agreement on six of seven domains or on all seven domains. The only patient-provider pair with low explanatory model agreement (one of seven) also had the lowest possible level of barrier, facilitator, and motivator agreement (no agreement on any of the three). Among the four patient-provider pairs with high explanatory model agreement, two had perfect barrier, facilitator, and motivator agreement and two agreed on two of the three. The only two other patient-provider pairs to achieve perfect barrier, facilitator, and motivator agreement achieved agreement on three and six of the seven domains in the explanatory model.
Discussion
This study had two goals: to examine the explanatory models that patients and their providers use to understand schizophrenia and to identify barriers, facilitators, and motivators associated with antipsychotic medication adherence from the patient perspective. Patients were able to provide meaningful responses to most questions about the explanatory model and about barriers, facilitators, and motivators.
Patients and their providers agreed completely on the name given to the patients' mental illness but had poor agreement on the cause of the illness and on how to recognize when the patient was doing well. Patients were more likely than providers to identify stress as an initial cause of their illness and as a factor that makes their illness worse, whereas providers were more likely to respond "I do not know" when asked to identify the cause of illness and to identify stopping medications as a factor that makes their patient's illness worse. There appears to be a mismatch between the patients' and providers' basic understanding of schizophrenia, which could have a negative impact on their therapeutic relationship and ultimately on patient adherence to treatment.
Consistent with other schizophrenia literature ( 34 ), in this study patients were more likely to identify improved functioning as evidence of a good outcome, whereas providers were more likely to cite decreased symptom severity. Patients' emphasis on functioning is consistent with the emphasis on recovery in the President's New Freedom Commission on Mental Health report ( 35 ) and does not focus on symptom management alone ( 35 , 36 ). Patients were also less likely than providers to identify stopping medications as causing their illness to get worse. If patients with schizophrenia do not associate medication nonadherence with a worsening of their illness and place a relatively low value on decreased symptoms, nonadherence to antipsychotic medication regimens is more understandable.
The 214 unique barrier, facilitator, and motivator items and 39 factors identified in this study are a coherent collection of medication adherence predictors that is consistent with the literature ( 11 , 37 , 38 , 39 , 40 ). As can be seen in the quotations in Tables 3 , 4 , and 5 , patients may struggle to clearly articulate specific barriers, facilitators, and motivators, but their responses are credible and consistent.
Of note is that the environment and provider-family relationship categories were the only ones that appeared as all three—barriers, facilitators, and motivators. This may reflect their importance to patient decisions; alternatively, these categories may simply be the most familiar to patients. More work is needed to determine the relative value of barriers, facilitators, and motivators for a given patient in order to target the ones that are most likely to influence adherence.
Even using a liberal definition of agreement, we found that more than one-third of the patient-provider pairs did not agree on barriers, facilitators, and motivators for patient medication adherence. The lowest agreement between patient and provider was about barriers. Given that some patients in this study had the same provider, one possible explanation for this finding is that the disagreement was centered within a few patient-provider pairs with the same provider. However, we did not find this to be the case. There were two patient-provider pairs who did not agree on any of the patient-identified barriers, facilitators, or motivators, and these patients were with different providers. Another possible explanation is that there would be more disagreement among pairs with a shorter clinical relationship. Again, this was not the case. If anything, the pairs with shorter clinical relationships were among those with the highest agreement.
Disagreement on medication adherence barriers, facilitators, and motivators can also contribute to medication nonadherence. At a minimum, if a provider focuses on a particular barrier, facilitator, or motivator that is not relevant to a patient, the provider's efforts to encourage adherence are likely to be ineffective. In addition, disagreement could have a negative impact on medication adherence via diminished patient-provider trust ( 41 , 42 , 43 ).
To bridge the gap between patient- and provider-identified barriers, facilitators, and motivators, we plan to refine and test a patient-centered medication adherence intervention for schizophrenia that incorporates the results from this study. We will use standard psychometric item-reduction strategies to create a barrier, facilitator, and motivator checklist that is more feasible for use under routine care conditions. We will address sustainability by combining these results with an options list specific to each patient-identified barrier, facilitator, and motivator for use by the provider within the clinical encounter. The above intervention components will be automated using a touch-screen computer interface.
Study limitations include the relatively small number of patients. Given the current lack of sustainable medication adherence interventions for patients with schizophrenia and the qualitative nature of this study, our goal was to achieve a deeper rather than a broader understanding of this important issue. All patients were taking their medication at the time of the interview, but for purposes of the interview all could easily recall a time when they were not medication adherent. We did not sample patients with a substance use disorder. We did not conduct the same type of open-ended interviews with providers as we did with patients. This may have increased patient-provider disagreement because providers might have provided more detail in an interview than they did responding to a paper-and-pencil survey. In the analysis phase, we used a liberal definition of agreement to at least partially account for this difference in data collection methods. In addition, whereas we sampled patients from both VA and non-VA settings and from both standard and intensive case-management programs in central Arkansas, inclusion of other locations and service delivery systems might have altered the scope of patient and provider responses.
Conclusions
We found substantial disagreement between patients and their providers with regard to their explanatory models for schizophrenia and limited provider understanding of the barriers, facilitators, and motivators affecting individual patients' medication adherence decisions. Our results highlight the need for tools to improve mental health providers' understanding of their patients' perspectives on schizophrenia and antipsychotic medication treatment. To that end, we will use the findings from this study to develop and evaluate a patient-centered intervention to enhance antipsychotic medication adherence.
Acknowledgments
This project was supported by Research Career Development Award RCD-97-310-2 from the Veterans Affairs Health Services Research and Development to Dr. Pyne and by the Department of Veterans Affairs South Central Mental Illness Research, Education, and Clinical Center.
1. Robins LN, Regier DA: Psychiatric Disorders in North America. New York, Free Press, 1991Google Scholar
2. Terkelsen KG, Menikoff A: Measuring the costs of schizophrenia: implications for the post-institutional era in the US. Pharmacoeconomics 8:199-222, 1995Google Scholar
3. Rice DP: The economic impact of schizophrenia. Journal of Clinical Psychiatry 60(suppl):4-6, 28-30, 1999Google Scholar
4. Cramer JA, Rosenheck R: Compliance with medication regimens for mental and physical disorders. Psychiatric Services 49:196-201, 1998Google Scholar
5. Lacro JP, Dunn LB, Dolder CR, et al: Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. Journal of Clinical Psychiatry 63:892-909, 2002Google Scholar
6. Valenstein M, Copeland LA, Blow FC, et al: Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Medical Care 40:630-639, 2002Google Scholar
7. Owen RR, Fischer EP, Booth BM, et al: Medication noncompliance and substance abuse among patients with schizophrenia. Psychiatric Services 47:853-858, 1996Google Scholar
8. Sullivan G, Wells KB, Morgenstern H, et al: Identifying modifiable risk factors for rehospitalization: a case-control study of seriously mentally ill persons in Mississippi. American Journal of Psychiatry 152:1749-1756, 1995Google Scholar
9. Weiden PJ, Olfson M: Cost of relapse in schizophrenia. Schizophrenia Bulletin 21:419-429, 1995Google Scholar
10. Gilbert PL, Harris MJ, McAdams LA, et al: Neuroleptic withdrawal in schizophrenic patients: a review of the literature. Archives of General Psychiatry 52:173-188, 1995Google Scholar
11. Fenton WS, Blyler CR, Heinssen RK: Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophrenia Bulletin 23:637-651, 1997Google Scholar
12. Grunebaum MF, Weiden PJ, Olfson M: Medication supervision and adherence of persons with psychotic disorders in residential treatment settings: a pilot study. Journal of Clinical Psychiatry 62:394-399, 2001 (Erratum 62:480, 2001)Google Scholar
13. Lehman AF, Carpenter WT Jr, Goldman HH, et al: Treatment outcomes in schizophrenia: implications for practice, policy, and research. Schizophrenia Bulletin 21:669-675, 1995Google Scholar
14. Lieberman JA, Stroup TS, McEvoy JP, et al: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209-1223, 2005Google Scholar
15. Zygmunt A, Olfson M, Boyer CA, et al: Interventions to improve medication adherence in schizophrenia. American Journal of Psychiatry 159:1653-1664, 2002Google Scholar
16. Boczkowski JA, Zeichner A, DeSanto N: Neuroleptic compliance among chronic schizophrenic outpatients: an intervention outcome report. Journal of Consulting and Clinical Psychology 53:666-671, 1985Google Scholar
17. Kemp R, Hayward P, Applewhaite G, et al: Compliance therapy in psychotic patients: randomised controlled trial (comment). British Medical Journal 312:345-349, 1996Google Scholar
18. O'Donnell C, Donohoe G, Sharkey L, et al: Compliance therapy: a randomised controlled trial in schizophrenia. British Medical Journal 327:834-838, 2003Google Scholar
19. Kelly GR, Scott JE: Medication compliance and health education among outpatients with chronic mental disorders. Medical Care 28:1181-1197, 1990Google Scholar
20. Bartholomew LK, Parcel GS, Kok G, et al: Changing behavior and environment: the foundation for changing health-related behavior, in Changing Patient Behavior: Improving Outcomes in Health and Disease Management. Edited by Patterson R. San Francisco, Jossey-Bass, 2001Google Scholar
21. Bartholomew LK, Parcel GS, Kok G: Intervention mapping: a process for developing theory- and evidence-based health education programs. Health Education Behavior 25:545-563, 1998Google Scholar
22. Happell B, Manias E, Roper C, et al: Wanting to be heard: mental health consumers' experiences of information about medication. International Journal of Mental Health Nursing 13:242-248, 2004Google Scholar
23. Carrick R, Mitchell A, Powell RA, et al: The quest for well-being: a qualitative study of the experience of taking antipsychotic medication. Psychological Psychotherapy 77:19-33, 2004Google Scholar
24. Weiden P, Rapkin B, Mott T, et al: Rating of Medication Influences (ROMI) scale in schizophrenia. Schizophrenia Bulletin 20:297-310, 1994Google Scholar
25. Corrigan PW, Liberman RP, Engel JD: From noncompliance to collaboration in the treatment of schizophrenia. Hospital and Community Psychiatry 41:1203-1211, 1990Google Scholar
26. Young JL, Spitz RT, Hillbrand M, et al: Medication adherence failure in schizophrenia: a forensic review of rates, reasons, treatments, and prospects. Journal of the American Academy of Psychiatry and the Law 27:426-444, 1999Google Scholar
27. Clark NM, Becker MH: Theoretical models and strategies for improving adherence and disease management, in The Handbook of Health Behavior Change. Edited by Shumaker SA, Schron EB, Ockene JK, et al. New York, Springer, 1998Google Scholar
28. Eraker SA, Kirscht JP, Becker MH: Understanding and improving patient compliance. Annals of Internal Medicine 100:258-268, 1984Google Scholar
29. Katzman R, Brown T, Fuld P, et al: Validation of a short Orientation-Memory-Concentration test of cognitive impairment. American Journal of Psychiatry 140:734-739, 1983Google Scholar
30. Kleinman A: Patients and Healers in the Context of Culture: An Exploration of the Borderland Between Anthropology, Medicine, and Psychiatry. Los Angeles, University of California Press, 1980Google Scholar
31. Kleinman A, Eisenberg L, Good B: Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Annals of Internal Medicine 88:251-258, 1978Google Scholar
32. Seidel J: The Ethnograph computer program. Littleton, Colo, Qualis Research Associates, 1998Google Scholar
33. Rodgers BL, Cowles KV: The qualitative research audit trail: a complex collection of documentation. Research in Nursing and Health 16:219-226, 1993Google Scholar
34. Fischer EP, Shumway M, Owen RR: Priorities of consumers, providers, and family members in the treatment of schizophrenia. Psychiatric Services 53:724-729, 2002Google Scholar
35. Achieving the Promise: Transforming Mental Health Care in America. Pub no SMA-03-3832. Rockville, Md, Department of Health and Human Services, President's New Freedom Commission on Mental Health, 2003Google Scholar
36. Liberman RP, Kopelowicz A: Recovery from schizophrenia: a concept in search of research. Psychiatric Services 56:735-742, 2005Google Scholar
37. Olfson M, Mechanic D, Hansell S, et al: Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatric Services 51:216-222, 2000Google Scholar
38. Perkins DO: Adherence to antipsychotic medications. Journal of Clinical Psychiatry 60:25-30, 1999Google Scholar
39. Garavan J, Browne S, Gervin M, et al: Compliance with neuroleptic medication in outpatients with schizophrenia; relationship to subjective response to neuroleptics; attitudes to medication and insight. Comprehensive Psychiatry 39:215-219, 1998Google Scholar
40. Kane JM: Compliance issues in outpatient treatment. Journal of Clinical Psychopharmacology 5(suppl):22S-27S, 1985Google Scholar
41. Dolder CR, Lacro JP, Leckband S, et al: Interventions to improve antipsychotic medication adherence: review of recent literature. Journal of Clinical Psychopharmacology 23:389-399, 2003Google Scholar
42. Lecompte D: Drug compliance and cognitive-behavioral therapy in schizophrenia. Acta Psychiatrica Belgica 95:91-100, 1995Google Scholar
43. Weiss M, Gaston L, Propst A, et al: The role of the alliance in the pharmacologic treatment of depression. Journal of Clinical Psychiatry 58:196-204, 1997Google Scholar



