Clozapine Treatment of Outpatients With Schizophrenia: Outcome and Long-Term Response Patterns
Editor's Note: Clozapine, an atypical neuroleptic, was approved by the U.S. Food and Drug Administration in 1990, marking a new era in the pharmacotherapy of schizophrenia. In the December 1993 issue of the journal, Alan Breier, M.D., and his colleagues at the Maryland Psychiatric Research Center reported on outcomes of 30 stable outpatients with schizophrenia treated with clozapine for one year. Eighteen patients showed sustained improvement in positive symptoms, with significantly fewer hospitalizations than in the year before treatment. Ninety-five percent of the clozapine responders were identified by the fourth month of treatment, and improvements in symptoms plateaued during the first six months. These findings helped establish early guidelines for clinicians making treatment decisions about this important new medication.
Objective: The purpose of the study was to examine the effects of clozapine in treating moderately ill schizophrenic outpatients and to determine the length of medication trial needed to identify responders and nonresponders. Methods: Rates of clinical response, relapses and hospitalizations, and levels of symptomatology and functioning were assessed for 30 chronic schizophrenic outpatients who received clozapine for one year. For some patients, data on relapse and hospitalization during treatment were compared with data from the year before treatment. Results: Eighteen of the 30 patients met criteria for sustained response; 17 of the responders were identified within the first four months of treatment. Patients experienced significantly fewer relapses and hospitalizations during treatment than in the previous year. Improvement in positive symptoms, generalsymptomatology, and levels of functioning reached a plateau during the first six months of treatment and remained at that level during the second six months. Negative symptoms and quality of life showed nonsignificant improvements at 12 months. Conclusions: Results support the use of clozapine in treating chronic, residually symptomatic schizophrenic outpatients. A four-month clozapine trial may be adequate to detect clinical responders in this population.
Clozapine is an atypical neuroleptic that has been shown in recent controlled studies (1,2) and descriptive studies (3,4,5) to have superior efficacy in treating severely ill treatment-resistant schizophrenic inpatients. It is the first agent in more than 40 years to be more effective than conventional neuroleptics in the treatment of schizophrenia, and it is marking a new era in the pharmacotherapy of this illness (6).
Since the U.S. Food and Drug Administration's approval of clozapine for treatment-resistant schizophrenia in 1990, its use has steadily grown. However, several questions related to its clinical use remain unanswered. Information about clozapine's effects in treating moderately ill, chronic outpatients is relatively scarce. The paucity of data is an important issue because approximately two-thirds of patients receiving clozapine are outpatients (Sandoz Pharmaceuticals, personal communication, May 1993), and it cannot be assumed that data collected on more severely ill inpatients generalizes to this group. Because the majority of schizophrenic patients are outpatients, it is important to acquire more data about clozapine's use in this population.
In addition, the length of the medication trial needed to identify responders and nonresponders has not been established, although trials of at least six months have been proposed (3,7). This issue should be resolved so that unwarranted clozapine risk and expense are avoided. A related point is knowing when progressive improvement ceases and plateauing of response occurs so that additional strategies, such as pharmacologic augmentation of clozapine treatment, may be considered. Other issues that have financial and clinical implications for outpatient care include effects on relapse and hospitalization rates.
In a recent double-blind parallel-groups clinical trial involving moderately ill chronic schizophrenic outpatients, we found that clozapine was superior to haloperidol in treating positive symptoms and had less clear-cut effects on negative symptoms (8). In the study reported here, we examined the response patterns of these patients to clozapine and their outcome over a one-year period. Sustained-response rates, relapse and hospitalization rates, and effects on symptoms and functioning were examined.
Methods
Admission criteria. Patients who participated in the study were stable outpatients in the Maryland Psychiatric Research Center outpatient program. Their ages ranged from 18 to 55 years. All patients met DSM-III-R criteria for chronic schizophrenia (9) as determined by the Structured Clinical Interview for DSM-III-R (SCID) (10) and a best-estimate diagnostic meeting led by two research psychiatrists. In addition, patients had a history of residual positive or negative symptoms after trials of conventional neuroleptics. The SCID was supplemented by past psychiatric records and data from available informants.
Patients with concurrent drug abuse, alcoholism, organic brain disorders, mental retardation, or a medical condition that is contraindicated for clozapine use were excluded from the study. Full admission criteria and details of the study design have been previously reported (8).
In brief, patients underwent an open six-week fluphenazine trial to ensure clinical stability and the existence of residual symptoms. They were randomly assigned to a ten-week parallel-groups double-blind comparison trial of clozapine or haloperidol. Following the double-blind trial, patients who were receiving clozapine were continued on open-labeled clozapine, and those receiving haloperidol were switched to open-labeled clozapine for a year-long descriptive study.
Throughout the study, all patients were seen weekly by their therapist. Medication compliance was assessed weekly by a pill count and weekly medication review. In addition, all patients had a compliance plan that consisted of medication checks by family or mental health professionals who had extensive contact with the patients.
The first patient entered the study in January 1990. At the time of this report, five patients were still in the final six months of the clozapine treatment period; data for these patients were included in the analyses.
Assessments. Positive symptoms were assessed by the sum of four items on the Brief Psychiatric Rating Scale (BPRS) (11): hallucinations, suspiciousness, disorganization, and bizarre thoughts. General symptoms were assessed by the total score on the 18-item BPRS. Negative symptoms were assessed by the total score on the Schedule for the Assessment of Negative Symptoms (SANS) (12). Functioning and quality of life were assessed using the Level of Functioning Scale (13) and Quality of Life Scale (14), respectively.
BPRS and SANS ratings were obtained weekly for the first ten weeks and then monthly for the remainder of the year-long outcome study. Assessments of functioning and quality of life were made at baseline (before clozapine treatment) and at six and 12 months. The symptom and functioning instruments were administered by master's-level and doctoral-level clinicians who had extensive experience with schizophrenia as well as clinical knowledge of the individual patients. Interrater reliability for these four instruments, as determined by interclass correlations, ranged from .76 to .90. Monthly interrater-reliability meetings were held throughout the study to minimize rater drift.
In addition, patient relapses were assessed each week for the 12-month period before the initiation of clozapine treatment and for the 12-month period of clozapine treatment. Relapse criteria were clinical judgment of symptomatic worsening and a change in BPRS rating from a stable baseline rating of 3 points on at least one of six critical items (hallucinations, suspiciousness, unusual thought content, conceptual disorganization, hostility, and somatic concerns) or a 3-point change in Clinical Global Index ratings (15). Hospitalization data were collected for the 12-month period after the initiation of clozapine treatment.
Responder criteria. Treatment response to clozapine was determined based on several criteria. We looked for a BPRS rating of positive symptoms that showed a 20 percent improvement over the baseline rating (before clozapine treatment), followed by a pattern of sustained improvement. A patient's improvement was judged to be sustained if at least 50 percent of subsequent BPRS ratings of positive symptoms met the 20 percent improvement (over baseline) criterion. The 20 percent BPRS symptom change criterion has been used in previous clozapine studies (1,2,3). The requirement of sustained improvement decreased the likelihood of identifying false positives secondary to spontaneous fluctuations in course.
Data analyses. Cumulative responder rates were determined using a life table analysis (16), with case inclusion occurring when the clinical responder criteria were met. Five patients were not included in this analysis because they did not meet the minimum positive-symptom criterion at baseline. Relapse and hospitalization data for the 12-month period before clozapine treatment were available for 21 patients who were enrolled in our clinic for at least a year before the clozapine trial. For five additional patients who were not enrolled for a full year before the trial, we reconstructed hospitalization data for the 12-month pretreatment period from patient records. Pretreatment relapse data were not available for these patients.
For the group of 21 patients, the frequency of contacts (weekly clinic visits) and the patient management approach (case management) were the same for the 12-month pretreatment period and the 12-month study period. Data on level of functioning and quality of life were not available for all subjects because these scales were added to the assessment battery after the study was initiated.
Chi square analysis was used to compare the number of patients experiencing relapses and hospitalizations during the 12 months before clozapine treatment and during the 12 months on clozapine. Relapse rates and number of hospitalizations and days hospitalized as well as symptom and functioning scores for the two periods were compared using paired t tests. Responder and nonresponder demographic and illness characteristics were compared using both chi square analysis and nonpaired t tests. All probability values were two tailed.
Results
Thirty-nine patients completed the ten-week double-blind phase (19 in the clozapine group and 20 in the haloperidol group) and entered the year-long descriptive study. Of these patients, four dropped out during the first six months of treatment; one patient had hepatic side effects, and four were noncompliant. The remaining 35 patients completed the first six months of treatment. Of these 35 patients, 30 completed one year of treatment and five were in the final six months of clozapine treatment at the time of this report.
The mean±SD age of the 35 patients was 34±7 years. Twenty-six patients were men, and nine were women. Twenty-six were white, and nine were African American. The mean±SD socioeconomic status as determined from the Hollingshead two-factor index was 4±1 (scored from 1 to 5). The mean length of illness for the 35 patients was 14.2±6 years, and the mean number of previous hospitalizations was 6.4±9. The mean±SD daily doses of clozapine at six and 12 months were 435.3±121 mg per day and 439.4±119 mg per day, respectively.
Sustained clinical improvement rates. As shown in Figure 1, of the 30 patients who completed one year of treatment, 60 percent (N=18) met criteria for sustained clinical improvement during the year. Seventeen of the 18 sustained responders (95 percent) were identified by the fourth treatment month (vertical dotted line). No significant differences were found between responders and nonresponders in gender, age at first psychotic symptoms, number of previous psychiatric hospitalizations, or daily dose of clozapine at outcome. There was, however, a trend (p=.07) for responders to be younger at first psychiatric hospitalization than nonresponders (mean±SD=20.4±5.6 years versus 25.6±7.3 years).
Relapses and hospitalizations. During the 12 months preceding clozapine treatment, 18 of the 21 patients for whom we had complete data experienced a relapse. Only four of these 21 patients had a relapse during the 12 months of clozapine treatment (χ2=12.1, df=1, p<.001). Of the 26 patients for whom we had complete hospitalization data, ten were hospitalized in the year before clozapine treatment, and two were hospitalized during the first year of treatment (χ2=6.1, df=1, p=.01).
As shown in Table 1, the mean number of relapses and the number of days in relapse were significantly reduced during clozapine treatment, as were the number of hospitalizations and days hospitalized.
Long-term response patterns.Table 2 presents data on symptom and functioning levels at baseline and six and 12 months after initiation of clozapine. BPRS ratings of positive symptoms, BPRS total symptom scores, and Level of Functioning Scale scores indicated significant improvement from baseline to six months; however, no significant differences between six- and 12-month ratings were found on these three measures. No significant improvements as measured by the SANS total score and the Quality of Life Scale were noted at six months, but trend effects were noted at 12 months. In contrast to the BPRS positive symptom ratings and total scores, which indicated that patients reached a plateau of improvement at six months, the Quality of Life Scale scores indicated steady improvement over the one-year period.
Discussion
In this sample of 30 patients who completed one year of clozapine treatment, 60 percent met the criterion for sustained clinical improvement at some point during the year. Other studies have reported clinically meaningful improvement rates of 30 percent to 61 percent (1,2,3,8). Differences in the findings of these studies may be related to the length of the clozapine trial, response criteria used, and characteristics of the samples. For example, in our ten-week double-blind study, 44 percent of clozapine-treated patients and only 5 percent of haloperidol-treated patients met the clinical response criterion (8). The 20 percent change in BPRS ratings of positive symptoms was used as a response criterion in both this study and the double-blind trial. However, because of the relatively short duration of the double-blind trial, the sustained-improvement criterion was not required.
The sustained-improvement criterion has advantages over a cross-sectional criterion because of the fluctuating nature of symptoms over time. Kane and associates (1) reported a 30 percent responder rate after only six weeks of treatment. Based on the improving trajectory of the six-week data, these investigators suggested that a longer trial may have resulted in higher improvement rates.
The sociodemographic and illness characteristics of the sample in our studies were reflective of chronic schizophrenic outpatients typically found in community mental health centers and university hospital clinics, with the exception that patients with concurrent drug abuse and alcoholism were excluded. Other studies included treatment-resistant inpatients.
Because of the associated financial expense and risk of agranulocytosis, it is important to develop clinical guidelines for the length of clozapine trial necessary to identify responders and nonresponders. The vast majority of responders in this study were identified early in the course of treatment. In fact, 95 percent of all responders were identified by the fourth month of treatment.
These data suggest that a four-month trial of clozapine may be adequate to distinguish responders and nonresponders. Meltzer and associates (3,7) have suggested that trials longer than six months may be required to distinguish responders from nonresponders. The discrepancy may be a result of these authors' tendency to exclude from their study patients who failed to demonstrate early symptom reductions, and thus they did not use a sample of consecutively treated patients. In our study, only one patient did not meet clinical improvement criteria until after the four-month mark; in that case, evidence of symptom reduction was clear before four months, suggesting that late clinical improvement in patients who have demonstrated no symptom change or minimal change during the first four months of clozapine treatment is rare.
Significantly fewer patients experienced relapses and hospitalizations during the first 12 months of clozapine treatment, compared with the 12-month pretreatment period in which patients were taking conventional neuroleptics. Moreover, the number of relapses and hospitalizations and days relapsed and hospitalized were reduced during clozapine treatment. Similarly, Meltzer and colleagues (3) reported a substantial reduction in rehospitalization rates of clozapine-treated patients.
The inability to control for changes secondary to the natural course of illness and differences in treatment limit the interpretation of pre- and posttreatment comparisons. This issue is somewhat mitigated in the study reported here because the patients had chronic unremitting illnesses. In addition, the patients included in the relapse analyses were receiving treatment in our clinic for the year before clozapine treatment and therefore had the same frequency of clinic contacts (weekly visits), had the same treatment approach (case management), and had the same frequency of compliance checks during the pre- and posttreatment periods. If confirmed in controlled prospective studies, these data have important budgetary and public health implications for the care of chronic schizophrenic patients.
Examination of behavioral data six and 12 months after clozapine was begun indicated that positive symptoms, general symptomatology, and level of functioning improved during the first six months of treatment. However, no significant improvements occurred from six to 12 months, suggesting that the trajectory of improvement may plateau before the second six months of clozapine treatment. Although the ratings were not made by clinicians blind to the treatment and therefore are limited, the interpretation of a plateauing response trajectory is probably valid because rater bias would likely favor progressive improvement from six to 12 months. If improvement does not progress beyond the first six months of treatment, additional pharmacologic or psychosocial interventions may be needed to augment benefits derived from clozapine therapy.
The effects on negative symptoms and quality of life measures were not statistically significant. The lack of a more robust negative-symptom response is consistent with data from our ten-week double-blind study (8) but is inconsistent with recent controlled studies reporting significant improvements in negative symptoms (1,2). Differences in patient populations, levels of psychopathology, and extrapyramidal symptoms at baseline are factors that may account for the discrepancies.
In contrast to the plateau pattern of improvement in positive and general symptoms, the trajectory of improvement in quality of life appeared linear throughout the year-long descriptive study. Perhaps improvement in quality of life occurs secondary to and as a result of symptom changes and therefore temporally follows symptom effects. This hypothesis is supported by other studies suggesting long-term progressive improvement in quality of life (3). More studies are needed to address this issue.
Conclusions
Sixty percent of patients met criteria for sustained clinical response within four months of treatment, suggesting that a four-month trial of clozapine is adequate to identify responders and nonresponders. The number of patients experiencing relapses and hospitalizations and the time spent in relapse and in the hospital were significantly curtailed during clozapine treatment. Improvements in symptoms, but not quality of life, plateaued during the first six months of treatment.
These data support the use of clozapine in treating chronic residually symptomatic schizophrenic outpatients. The wider use of clozapine and the development of new antipsychotic agents with improved efficacy and side-effect profiles may have major clinical and public health impacts on the care of schizophrenic patients.
Acknowledgments
The authors acknowledge the contributions of Brian Kirkpatrick, M.D., Marlene Shapiro, M.S.W., Pat Ball, B.S.N., and other clinical and research staff of the Maryland Psychiatric Research Center; Diane Brandt, B.S., for data management; Ann Summerfelt and Kevin O'Grady, Ph.D., for statistical consultation; and Jane Hoffmann for secretarial assistance. This work was supported by grants MH45074 and MH40279 from the National Institute of Mental Health.
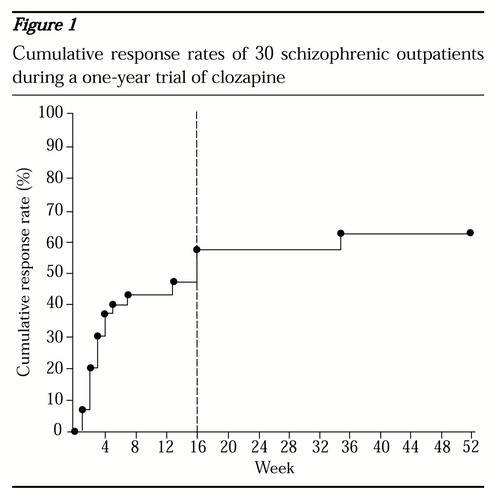
Figure 1. Cumulative response rates of 30 schizophrenic outpatients during a one-year trial of clozapine
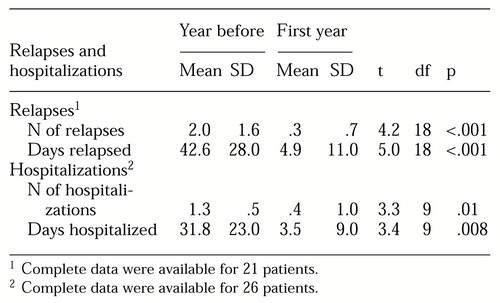 |
Table 1. Relapses and hospitalizations among schizophrenic outpatients during the year before and the first year of clozapine treatment
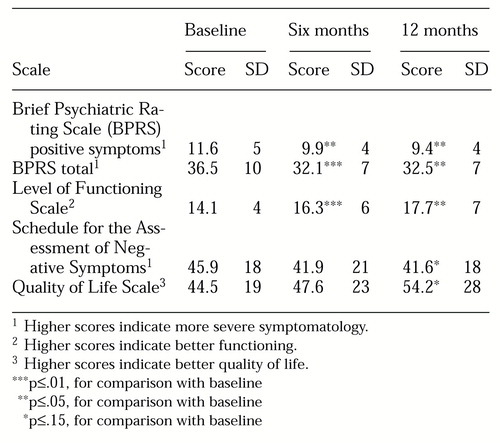 |
Table 2. Mean scores on scales assessing symptoms, functioning, and quality of life of schizophrenic outpatients before clozapine treatment (baseline) and at six and 12 months of treatment
1. Kane J, Honigfeld G, Singer J, et al: Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Archives of General Psychiatry 45:789-796, 1988Crossref, Medline, Google Scholar
2. Pickar D, Owen RR, Litman RE, et al: Clinical and biologic response to clozapine in patients with schizophrenia: crossover comparison with fluphenazine. Archives of General Psychiatry 49:345-353, 1992Crossref, Medline, Google Scholar
3. Meltzer HY, Bernett S, Bastani B, et al: Effects of six months of clozapine treatment on the quality of life of chronic schizophrenic patients. Hospital and Community Psychiatry 41:892-897, 1990Abstract, Google Scholar
4. Davies MA, Conley RR, Schulz SC, et al: One-year follow-up of 24 patients in a clinical trial of clozapine. Hospital and Community Psychiatry 42:628-629, 1991Abstract, Google Scholar
5. Wilson WH: Clinical review of clozapine treatment in a state hospital. Hospital and Community Psychiatry 43:700-703, 1992Abstract, Google Scholar
6. Breier A, Buchanan RW, Kirkpatrick B, et al: Clozapine: current status and future directions. Presented at the annual meeting of the American Psychiatric Association, San Francisco, May 22-27, 1993Google Scholar
7. Meltzer HY, Bastani B, Young KK, et al: A prospective study of clozapine in treatment-resistant schizophrenic patients: I. preliminary report. Psychopharmacology 99(suppl):68-72, 1989Google Scholar
8. Breier A, Buchanan RW, Kirkpatrick B, et al: Clozapine in schizophrenic outpatients: effects on positive and negative symptoms. American Journal of Psychiatry, in pressGoogle Scholar
9. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed, rev. Washington, DC, American Psychiatric Association, 1987Google Scholar
10. Spitzer RL, Williams JBW, Gibbon N, et al: Structured Clinical Interview for DSM-III-R. New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
11. Overall JE, Gorham DE: The Brief Psychiatric Rating Scale. Psychology Reports 10:799-812, 1961Google Scholar
12. Andreasen NC: Negative symptoms in schizophrenia. Archives of General Psychiatry 39:784-788, 1982Crossref, Medline, Google Scholar
13. Hawk AB, Carpenter WT, Strauss JS: Diagnostic criteria and five-year outcome in schizophrenia: a report from the International Pilot Study of Schizophrenia. Archives of General Psychiatry 32:343-347, 1975Crossref, Medline, Google Scholar
14. Heinrichs DW, Hanlon TE, Carpenter WT: The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin 10:388-398, 1984Crossref, Medline, Google Scholar
15. Guy W: ECDEU Assessment Manual for Psychopharmacology. DHHS pub (ADM) 76-338. Rockville, Md, National Institute of Mental Health, 1976Google Scholar
16. Kalbfleisch JD, Prentice RL: The Statistical Analysis of Failure Time Data. New York, Wiley, 1980Google Scholar



