MOUD Adoption Among Clients of Organizations That Provide MOUD or Coordinate Care With External Providers
In July 2018, Philadelphia became the first U.S. municipality to mandate the availability of medications for opioid use disorder (MOUD) in publicly funded substance use disorder treatment agencies. All agencies were required to provide MOUD onsite or document a plan to coordinate care with external MOUD providers (1). Although the latter option allowed agencies to comply with the mandate without dispensing MOUD themselves, loss to follow-up has been documented in other systems that coordinate external care for opioid use disorder (2, 3). We compared rates of MOUD receipt among individuals with opioid use disorder who were able to obtain MOUD onsite at their substance use disorder treatment agency versus those who had to obtain MOUD from external providers.
From March to July 2020, we recruited leaders from all opioid use disorder treatment agencies in Philadelphia. Leaders from 45 of 53 agencies (an 85% response rate) reported which types of MOUD their agencies provide and whether they dispense or prescribe MOUD onsite or coordinate with external providers. Using 2019 Medicaid data, we calculated the proportion of individuals with opioid use disorder who received full (methadone) or partial (buprenorphine) opioid agonists. We used Wilcoxon rank sum tests to compare receipt rates of opioid agonist–type MOUD among clients of agencies that did versus did not provide onsite options for obtaining MOUD agonists. Although individuals’ uptake of opioid antagonist–type MOUD is low (average of <5% across the four quarters of 2019) in the publicly funded system in Philadelphia, we also compared rates of naltrexone receipt across groups. The institutional review boards of the University of Pennsylvania and the City of Philadelphia approved the study procedures and the use of Medicaid data.
Most leaders (N=34, 76%) reported that their agency dispenses or prescribes at least one MOUD agonist onsite. The median proportion of clients taking a MOUD agonist was significantly higher for onsite providers than for agencies that referred their clients to external MOUD providers (43% vs. 28%, p=0.011) (Figure 1). Leaders of agencies that provided MOUD agonists onsite were also more likely to report onsite naltrexone provision (N=24 of 34, 71% vs. N=2 of 11, 18%, p=0.002), suggesting that antagonist provision did not offset low agonist provision.
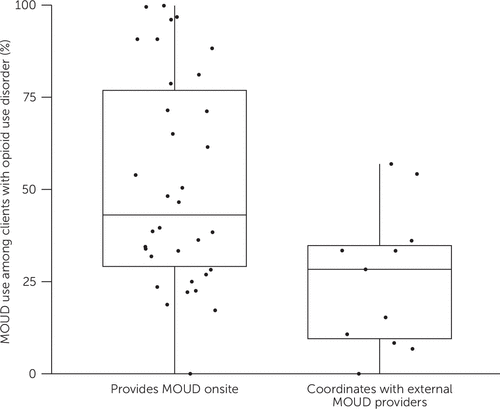
FIGURE 1. Clients’ receipt of opioid agonist–type medications for opioid use disorder (MOUD), by agency typea
aHorizontal lines within boxes represent median values, and dots represent individual leaders. The median percentage of clients taking MOUD was significantly higher for onsite versus offsite MOUD provision (p=0.011).
Although our study had limitations, including potential error in agency directors’ reporting and a correlational design, the findings have important implications. Agencies that provide MOUD onsite had a higher median proportion—by almost 15 percentage points—of clients receiving these lifesaving medications relative to agencies that coordinate care with external providers. Agencies that coordinate with external MOUD providers may be creating barriers that impede uptake, or the clients of these agencies may be less motivated to engage with MOUD treatment. Regardless, these findings highlight the need for MOUD mandates specifying how care is provided at substance use disorder treatment agencies, as well as for efforts to build the capacity of these agencies to provide MOUD onsite.
1. : Care coordination between rural primary care and telemedicine to expand medication treatment for opioid use disorder: results from a single‐arm, multisite feasibility study. J Rural Health 2023; 39:780–788Crossref, Medline, Google Scholar
2. : A scalable, automated warm handoff from the emergency department to community sites offering continued medication for opioid use disorder: lessons learned from the EMBED trial stakeholders. J Subst Abuse Treat 2019; 102:47–52Crossref, Medline, Google Scholar
3. : Developing a cascade of care for opioid use disorder among individuals in jail. J Subst Abuse Treat 2022; 138:108751Crossref, Medline, Google Scholar


