Breast Cancer Screening Rates Among Medicaid Beneficiaries With Schizophrenia
Abstract
Objective:
Women with serious mental illness are more likely to be diagnosed as having late-stage breast cancer than women without serious mental illness, suggesting a disparity in screening mammography. This study aimed to compare screening mammography rates in a nationally representative sample of Medicaid beneficiaries with and without schizophrenia.
Methods:
Medicaid Analytic eXtract files, 2007–2012, were used to identify a cohort of women ages 40–64 with schizophrenia who were eligible for Medicaid but not Medicare (N=87,572 in 2007 and N=114,341 in 2012) and a cohort without schizophrenia, frequency-matched by age, race-ethnicity, and state (N=97,003 in 2007 and N=126,461 in 2012). Annual screening mammography rates were calculated and adjusted for demographic characteristics and comorbid conditions. Multivariable logistic regression was used to estimate the association between beneficiary characteristics and screening mammography rates.
Results:
In 2012, 27.2% of women with schizophrenia completed screening mammography, compared with 26.8% of the control cohort. In the schizophrenia cohort, American Indian/Alaskan Native women had significantly lower odds of receiving mammography (OR=0.82, p=0.02) than White women, whereas Hispanic/Latina women had higher odds (OR=1.16, p<0.001). Women with schizophrenia and a nonalcohol-related substance use disorder had lower odds of receiving mammography (OR=0.74, p<0.001) than women without a substance use disorder. Having at least one medical visit in the past year (vs. no visits) increased the odds of receiving screening mammography (OR=5.08, p<0.001).
Conclusions:
Screening mammography rates were similar between Medicaid-insured women with and those without schizophrenia. Interventions to increase uptake may need to focus on improving socioeconomic conditions and primary care engagement for vulnerable populations, regardless of psychiatric condition.
HIGHLIGHTS
Among Medicaid beneficiaries in 2007–2012, women with schizophrenia had screening mammography rates that were similar to those among women without schizophrenia.
For women with schizophrenia, having at least one medical visit in the past year had the strongest association with receiving screening mammography among the variables studied.
Women with schizophrenia and comorbid substance use disorders were less likely than women without these conditions to receive screening mammography.
Breast cancer affects one in eight American women during their lifetimes, and screening mammography is the main early-detection tool (1). Previous studies have found that women with serious mental illness, such as schizophrenia, are less likely to receive guideline-concordant screening mammography than women in the general population (2, 3). This care gap contributes to women with serious mental illness being diagnosed more frequently as having later-stage tumors and more advanced lymph node spread than women without serious mental illness (4, 5). Thus, delayed breast cancer diagnoses contribute to increased morbidity and mortality rates among women with serious mental illness (6–9).
A recent systematic review including studies from 11 countries found that women with schizophrenia were half as likely to receive screening mammography as their peers without schizophrenia (2). However, most of the reviewed studies had small sample sizes, focused on a single city or region, or lacked an appropriate comparable control group. Larger studies have more commonly focused on women with any mental illness (10), an approach that may obscure care gaps specific to women with serious mental illness. Furthermore, some studies have relied on self-reported breast cancer–screening data, which tend to overreport screening compared with medical claims (11–13).
Several hypotheses have been proposed to explain this screening gap. First, people with higher socioeconomic status and those with commercial insurance are more likely to receive breast cancer screening (14). These factors could account for the observed difference in screening rates, given that adults with schizophrenia are more likely than those without schizophrenia to have low incomes and public insurance (15). Second, people with schizophrenia may be less likely than their peers to engage in preventive care, such as screening mammography (16). Finally, people with schizophrenia may encounter cognitive or behavioral challenges while navigating complex health care systems (17, 18). At the health systems level, stigma may interfere with timely referral to screening mammography, akin to other types of delayed treatment (19, 20). For example, providers may not prioritize cancer screening or may worry about the ability of people with schizophrenia to follow up with care (21, 22).
To build on previous work, in this study we restricted the sample to Medicaid beneficiaries, included a frequency-matched control cohort, and provided objective information about health service utilization. This rigorous approach addressed a gap in the literature by accounting for health insurance status and health service utilization to parse the mechanisms underlying low breast cancer screening rates among women with schizophrenia.
To evaluate long-term trends in screening mammography, we used administrative claims to conduct a longitudinal, nationwide study of women with schizophrenia in the United States. We aimed to estimate the annual rates of screening mammography among Medicaid recipients with and those without schizophrenia, evaluate variation in screening rates by schizophrenia diagnosis across U.S. states, and examine patient-level risk factors for screening among women with schizophrenia.
Methods
Study Sample and Design
In this retrospective cohort study, we compared screening mammography among women with schizophrenia with a frequency-matched 1:1 cohort of women without schizophrenia, matched by age and race-ethnicity upon first entry into the cohort, as described previously (23). Data were from a parent study that used Centers for Medicare and Medicaid Services Medicaid Analytic eXtract files, which provide a nationally representative sample of Medicaid beneficiaries. However, data availability restricted the parent sample to Medicaid beneficiaries enrolled between January 1, 2002, and December 31, 2012.
For the parent study, the inclusion criteria for all participants included living in one of the 41 states with available data, with at least 11 months of eligibility during the calendar year. Participants in the cohort with schizophrenia included women with one or more inpatient claims for schizophrenia (ICD-9 code 295.x) or two or more outpatient claims for schizophrenia within any 6-month period (24). Participants in the control group had no schizophrenia claims nor any diagnoses of bipolar disorder (ICD-9 296.0x, 296.4x–296.7x, 296.80, or 301.13), psychosis (ICD-9 298.x), delusional disorders (ICD-9 297.x), or pervasive developmental disorder (ICD-9 299.x) during the entire study period. Individuals remained in their respective cohorts in each year of the 2002–2012 study period in which they continued to meet eligibility criteria. Differences in size between the two cohorts were possible because this study included only non–dually eligible Medicaid beneficiaries (i.e., eligible for Medicaid but not for Medicare). Institutional review board approval was waived because the study used deidentified data.
The subset of the cohort from the parent study studied here included women ages 40–64 years and was restricted to the years 2007–2012 because of changes in screening mammography claims coding that started in 2007. Data from participants with a known breast cancer diagnosis (ICD-9 174*, 233*, and V10.3) 1 year before the observed year were excluded. The selected age range accounted for variations in guidelines regarding the earliest age for screening mammography. Whereas the American Cancer Society recommends that women begin biennial screening mammography at age 40 (25), the U.S. Preventive Services Task Force recommends regular screening starting at age 50 (1).
Outcome Assessment
The primary outcome was at least one screening mammography procedure (CPT code 77051, 77052, 77055, 77056, 77057, or G0202) assessed dichotomously (i.e., ≥1 vs. 0) in every year of the study period, following previously validated methods (26, 27). Annual screening rates are typically expected to be lower than guideline-recommended biennial rates but can be used for comparison, and this analytic approach is common for studying screening mammography.
Covariates
All covariates were coded categorically. Nominally assessed covariates included race-ethnicity (non-Hispanic White, non-Hispanic Black, American Indian/Alaskan Native, Asian, Hispanic/Latina, Native Hawaiian/other Pacific Islander, multiracial, or unknown), substance use disorder (alcohol, opioid, cocaine, cannabis, or other), other mental disorders (anxiety or depression), medical conditions (diabetes mellitus, hypertension, or dyslipidemia), and health care utilization (medical care and mental health care visits).
Statistical Analysis
Individual characteristics were summarized by using frequencies and chi-square tests of homogeneity to compare the beneficiaries in the schizophrenia and control cohorts. Annual mammography screening rates were calculated for each cohort. To account for clustering within strata, all models were adjusted for matching factors, including age and race-ethnicity. Models were also adjusted for diagnoses of substance use disorders, other mental disorders, and medical conditions. Within the schizophrenia cohort, we used multivariable logistic regression to estimate associations between beneficiary characteristics (demographic characteristics, comorbid conditions, and health care utilization) and the likelihood of receiving screening mammography annually, with robust standard errors to account for repeated outcome measurements in the longitudinal cohort. Given variations in screening mammography guidelines (1, 25), we conducted a sensitivity analysis including only patients ages 50–64 to assess the robustness of our study findings. We used Stata, version 16, for statistical analyses.
Results
Table 1 shows the demographic characteristics, comorbid conditions, and health care utilization rates for the two cohorts. The schizophrenia cohort included 87,572 women in 2007 and 114,341 in 2012. The control cohort included 97,003 women in 2007 and 126,461 in 2012.
| 2007 (N=184,575) | 2012 (N=240,802) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia cohort (N=87,572) | Control cohort (N=97,003) | Schizophrenia cohort (N=114,341) | Control cohort (N=126,461) | |||||||
| Characteristic | N | % | N | % | p | N | % | N | % | p |
| Age (M±SD years) | 51.9±6.6 | 51.8±6.9 | .001 | 52.7±6.6 | 51.9±6.9 | <.001 | ||||
| Race | <.001 | <.001 | ||||||||
| Non-Hispanic White | 37,011 | 42.3 | 45,710 | 47.1 | 44,663 | 39.1 | 56,129 | 44.4 | ||
| Non-Hispanic Black | 30,906 | 35.3 | 30,938 | 31.9 | 41,853 | 36.6 | 41,163 | 32.6 | ||
| American Indian/Alaskan Native | 663 | .8 | 781 | .8 | 912 | .8 | 1,160 | .9 | ||
| Asian | 1,546 | 1.8 | 2,529 | 2.6 | 2,248 | 2.0 | 3,410 | 2.7 | ||
| Multiracial | 178 | .2 | 149 | .2 | 309 | .3 | 329 | .3 | ||
| Hispanic/Latina | 7,897 | 9.0 | 9,406 | 9.7 | 11,948 | 10.5 | 13,161 | 10.4 | ||
| Native Hawaiian/other Pacific Islander | 1,381 | 1.6 | 1,170 | 1.2 | 1,717 | 1.5 | 1,393 | 1.1 | ||
| Unknown | 7,990 | 9.1 | 6,320 | 6.5 | 10,691 | 9.4 | 9,716 | 7.7 | ||
| Comorbid condition | ||||||||||
| Medical disorder | ||||||||||
| Hypertension | 40,828 | 46.6 | 36,089 | 37.2 | <.001 | 58,196 | 50.9 | 47,487 | 37.6 | <.001 |
| Dyslipidemia | 27,919 | 30.7 | 23,668 | 22.2 | <.001 | 38,566 | 33.7 | 31,748 | 25.1 | <.001 |
| Diabetes mellitus | 27,836 | 31.8 | 20,130 | 20.8 | <.001 | 37,974 | 33.2 | 25,163 | 19.9 | <.001 |
| Substance use disorder | ||||||||||
| Alcohol | 3,717 | 4.2 | 1,027 | 1.1 | <.001 | 5,276 | 4.6 | 1,482 | 1.2 | <.001 |
| Other (nonalcohol) substance use | 19,018 | 21.7 | 8,171 | 21.7 | <.001 | 32,141 | 28.1 | 14,470 | 11.4 | <.001 |
| Psychiatric disorder | ||||||||||
| Anxiety | 9,906 | 11.3 | 7,043 | 7.3 | <.001 | 20,523 | 17.8 | 12,723 | 10.1 | <.001 |
| Depression | 24,721 | 28.2 | 13,056 | 13.5 | <.001 | 35,682 | 31.2 | 18,613 | 14.7 | <.001 |
| Health care utilization | ||||||||||
| ≥1 general medical visits | 64,708 | 73.9 | 68,409 | 70.5 | <.001 | 90,040 | 78.8 | 92,226 | 72.9 | <.001 |
| ≥1 mental health visits | 43,017 | 49.1 | 5,382 | 5.6 | <.001 | 56,643 | 49.5 | 8,979 | 7.1 | <.001 |
TABLE 1. Characteristics of women with schizophrenia and a frequency-matched control cohort, 2007–2012 (N=425,377)
Women with schizophrenia had higher rates of co-occurring alcohol and other substance use disorders, anxiety, depression, hypertension, dyslipidemia, and diabetes compared with the control cohort. In addition, women with schizophrenia were more likely to have had both medical and mental health outpatient visits within a given year compared with women in the control cohort.
Figure 1 shows the unadjusted annual screening mammography rates for women with or without schizophrenia. (See an online supplement to this article for additional details.) The screening mammography rate in 2007 was 22.2% for both cohorts (schizophrenia, N=19,445; control, N=21,522), and in 2012 was 27.2% (N=31,061) for the schizophrenia cohort and 26.8% (N=33,895) for the control cohort. Both groups had increasing rates of completed mammography screening during the 2007–2012 study period, an increase of 5.0 percentage points (22.2%, N=19,445 to 27.2%, N=31,061) and 4.6 percentage points (22.2%, N=21,522 to 26.8%, N=33,895), respectively, for the schizophrenia and control cohorts. The largest annual rate difference between the cohorts was 0.9 percentage points in 2008 (p<0.001). Sensitivity analysis restricting the sample to women ages 50–64 did not alter the findings.
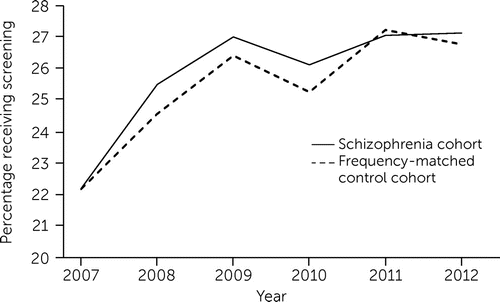
FIGURE 1. Screening mammography rates for women with schizophrenia and a frequency-matched control cohort, 2007–2012a
aUnadjusted annual mammography rate was defined as at least one claim for screening mammography in a given year per non–dually eligible Medicaid beneficiary, divided by the number of non–dually eligible beneficiaries.
Figure 2 shows ORs for screening mammography for women in the schizophrenia cohort in 2012, by demographic variables, comorbid conditions, and health care utilization. Among women with schizophrenia, those of American Indian or Alaskan Native race-ethnicity had significantly lower odds of receiving mammography (OR=0.82, 95% CI=0.70–0.97, p=0.02) than White women, whereas those of Hispanic or Latina ethnicity had higher odds of completion (OR=1.16, 95% CI=1.11–1.21, p<0.001). Odds of completing breast cancer screening also varied by age. Compared with women ages 60–64 years, women ages 40–49 had significantly lower odds of receiving mammography (OR=0.89, 95% CI=0.85–0.92, p<0.001), whereas women ages 50–59 had slightly higher odds (OR=1.05, 95% CI=1.01–1.09, p=0.02). Women with a comorbid diagnosis of any substance use disorder, including alcohol use disorder, had lower odds of receiving mammography (alcohol: OR=0.81, 95% CI=0.76–0.87, p<0.001; other substance use: OR=0.74, 95% CI=0.72–0.77, p<0.001) than women without a substance use disorder. Anxiety also was associated with lower odds of receiving mammography (OR=0.86, 95% CI=0.83–0.89, p<0.001) than having no diagnosis. Diagnoses of hypertension and dyslipidemia were associated with greater odds of receiving mammography (hypertension: OR=1.07, 95% CI=1.04–1.10, p<0.001; dyslipidemia: OR=1.54, 95% CI=1.50–1.59, p<0.001) compared with having no diagnosis.
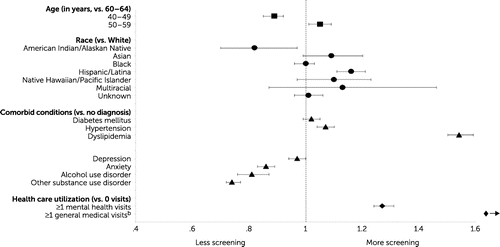
FIGURE 2. Screening mammography among women with schizophrenia, by demographic characteristics, comorbid conditions, and health care utilization, 2012a
aMultivariable logistic regression with robust standard errors was used to estimate associations between patient characteristics and the likelihood of receiving screening mammography; values on the x-axis denote ORs.
bOR=5.08, 95% CI=4.84–5.33.
Finally, among the factors studied, higher health care utilization was associated with the greatest odds of receiving mammography. Women with schizophrenia who had at least one medical visit in the past year had more than five times higher odds of receiving breast cancer screening than women with schizophrenia without a medical visit (OR=5.08, 95% CI=4.84–5.33, p<0.001). Women with schizophrenia who had at least one mental health visit in the year had higher odds of receiving mammography compared with women with schizophrenia without any mental health visits that year (OR=1.27, 95% CI=1.24–1.31, p<0.001).
Figure 3 shows the geographic distribution of mammography screening rates among women with schizophrenia by state for 2012. The rates (adjusted for age, race-ethnicity, and comorbid conditions) ranged from 12.2% in Hawaii to 38.1% in Massachusetts.
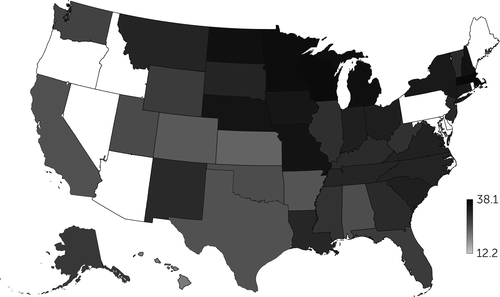
FIGURE 3. Screening mammography rates for women with schizophrenia, by statea
aShading indicates the percentage of non–dually eligible Medicaid beneficiaries with schizophrenia in the state who received at least one screening mammography in 2012 (N=31,061). Screening rates were adjusted for age, race-ethnicity, and comorbid conditions. Data were unavailable for states shown in white.
Discussion
To our knowledge, this is the first longitudinal, nationwide study of screening mammography rates of women with schizophrenia. Unlike previous research that has reported marked disparities in screening mammography between women with and women without schizophrenia (28), we found only a minimal difference in screening mammography rates between the two groups of women who were publicly insured. Although this difference reached statistical significance, the two rates differed by <1 percentage point, below the 5-percentage-point difference serving as the cutoff for a clinically relevant disparity (29). In addition, women with schizophrenia often had higher screening rates than women in the control group, an observation that was the opposite of previous findings. These findings suggest that previously reported lower rates of breast cancer screening among women with schizophrenia were likely driven primarily by lower socioeconomic status and insurance status.
Among women with schizophrenia, having at least one medical visit within the year was the strongest predictor of receiving mammography, consistent with results from multiple previous studies (28, 30, 31). This finding may help explain in part why comorbid conditions such as dyslipidemia and hypertension increased the likelihood of receiving mammography: patients with schizophrenia and comorbid cardiovascular disease may be more likely to be seen by primary care providers, which, in turn, increases the likelihood of being referred to other health screenings. On the other hand, women with schizophrenia who seek general medical care (such as cardiovascular care) may also be more motivated to complete cancer screenings. Our finding suggests that connecting individuals with schizophrenia with a primary care provider may have the greatest impact on their access to cancer screening and perhaps other preventive care services. More research is needed to understand patient- and provider-level drivers underlying completion of screening mammography.
In addition, women with schizophrenia who also have substance use disorders or who identify as American Indian/Alaskan Native were less likely, compared with women without such disorders and White women, respectively, to receive mammography. These groups may benefit from additional types of support and targeted interventions to complete screening. Hispanic/Latina women with schizophrenia were more likely than White women with schizophrenia to complete screening, contrasting findings from previous studies of racial-ethnic disparities in utilization of screening mammography, which may be related to insurance coverage for this population (32).
Our study found that women with Medicaid—regardless of psychiatric comorbidity— had much lower mammography screening across all years (N=309,032 of 1,201,280, 25.7%), compared with women with commercial insurance in previous studies (54.7%) (33). This finding suggests that having health insurance coverage may be necessary, but is not sufficient, for ensuring adequate mammography screening. Social determinants of health such as poverty, access to transportation, and safe housing may also be important factors to support women in meeting their health care needs. To improve breast cancer screening rates for women with schizophrenia, attention may need to shift to policy solutions that address structural racism, neighborhood context, and environmental factors (34, 35). Social determinants of health are likely also key drivers of morbidity and mortality gaps among people with serious mental illness, indicating the need for multilevel interventions to address structural, provider-related, and individual change (36).
We also found considerable state-level variation in mammography screening for people with schizophrenia, with annual rates ranging from 12% to 38%. These findings warrant further examination because they may highlight that specific state programs improve the integration of primary care and behavioral health services. For example, New York State increased screening by >10% for women with schizophrenia from 2007 to 2012, compared with a 6.5% increase for the control cohort. New York State runs the Cancer Services Program, which aims to increase cancer screenings, and has launched a series of collaborative care projects focused on improving health for people with serious mental illness (37, 38).
Another example is the state of Missouri, where the screening mammography rate was 33.1% for women with schizophrenia and 24.0% for women in the control cohort in 2012. In 2008, Missouri launched a pilot program to enhance preventive medical care for Medicaid beneficiaries with serious mental illness (39, 40). This program later became a statewide initiative, establishing “health homes” throughout the state’s community mental health centers to integrate primary care and mental health services. Although the health homes emphasized metabolic screening as a key primary care measure, access to other types of screening tests, such as mammography, may have improved as well.
Nationally, state mental health commissioners have prioritized and developed metrics for well-being in integrated mental health and primary care (40). Much of the focus has been on cardiovascular care for people with serious mental illness, given the adverse effects of second-generation antipsychotic medications and the high prevalence of metabolic syndrome among individuals with serious mental illness (41). However, cancer-related morbidity is an often-overlooked health disparity for individuals with serious mental illness (42). To ensure a broad panel of key preventive care screenings, policy makers should consider including breast cancer and other cancer screenings in these measurements of health care delivery for people with schizophrenia and other serious mental illness, given the low screening rates in this population (4, 5).
This study had several limitations. Although case-control matching accounted for confounding by age and race-ethnicity, unmeasured variables included housing status and smoking history. The methods for selecting the schizophrenia cohort may have missed women who primarily receive crisis care, and the selection was not limited to women taking prolactin-raising antipsychotics, which are associated with increased breast cancer risk (43). This study excluded dually eligible Medicaid and Medicare beneficiaries, who represent a vulnerable population with a high need for complex care. The study sample also did not reflect uninsured, privately insured, or incarcerated populations. In addition, claims data were limited by the quality of submitted claims, and variability may have existed across states. Furthermore, because of processing time, these claims were several years old and preceded Medicaid expansion under the Affordable Care Act, starting in 2014, which broadened access to screening mammography and established models of care that focused on integrating general medical and behavioral health services, including health home models and accountable care organizations (44). The study period also preceded the COVID-19 pandemic, which may have affected preventive care delivery for this population. Evaluating the outcomes of these initiatives and service disruptions will be critical next steps.
Study strengths included the large, frequency-matched sample with longitudinal outcomes data, which captured important trends in policy changes affecting Medicaid-insured women in the United States during this period. This study provided a baseline for comparison for future studies to examine the effects of Medicaid expansion on screening mammography for women with schizophrenia. Additional studies should also examine differences between Medicaid-only beneficiaries and dually eligible Medicaid and Medicare beneficiaries, because dually eligible recipients are more likely to have multiple chronic conditions, which may affect their access to preventive care (45). Although dually eligible beneficiaries tend to have lower incomes than Medicaid-only recipients, the higher reimbursement for health services through Medicare could increase their uptake of mammography (46). Finally, screening mammography is only the first step in cancer care, so future work is needed to focus on the stage of cancer at diagnosis, presence of follow-up, and adherence to guideline-concordant breast cancer treatment for women with schizophrenia.
Conclusions
In a national cohort of non–dually eligible Medicaid beneficiaries, almost 75% of women did not receive annual screening mammography. Although screening mammography rates appeared to be comparable between publicly insured women with and women without schizophrenia, they were overall much lower than guidelines recommend. We found wide state-level variation in mammography screening rates for women with schizophrenia, and this variation should be examined further to identify promising programs. We also found that women with health system engagement had significantly higher rates of mammography screening, suggesting that efforts to improve health care integration may improve breast cancer detection, and therefore health outcomes, in this high-priority population.
1. : Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726Crossref, Medline, Google Scholar
2. : Breast cancer screening in women with schizophrenia: a systematic review and meta-analysis. Psychiatr Serv 2020; 71:263–268Link, Google Scholar
3. : Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry 2014; 205:428–435Crossref, Medline, Google Scholar
4.
5. : Improving breast cancer screening and care for women with severe mental illness. J Clin Oncol 2017; 35:3996–3998Crossref, Medline, Google Scholar
6. : Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015; 72:1172–1181Crossref, Medline, Google Scholar
7. : A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007; 64:1123–1131Crossref, Medline, Google Scholar
8. : Mortality in schizophrenia: 30-year nationwide follow-up study. Acta Psychiatr Scand 2018; 138:492–499Crossref, Medline, Google Scholar
9. : Increasing mortality gap for patients diagnosed with schizophrenia over the last three decades—a Danish nationwide study from 1980 to 2010. Schizophr Res 2013; 146:22–27Crossref, Medline, Google Scholar
10. : Disparities in breast cancer screening uptake for women with mental illness in the United Kingdom. Am J Prev Med 2021; 60:e123–e130Crossref, Medline, Google Scholar
11. : Bias associated with self-report of prior screening mammography. Cancer Epidemiol Biomarkers Prev 2009; 18:1699–1705Crossref, Medline, Google Scholar
12. : Validating self-reported mammography use in vulnerable communities: findings and recommendations. Cancer Epidemiol Biomarkers Prev 2014; 23:1649–1658Crossref, Medline, Google Scholar
13. : Mammography status using patient self-reports and computerized radiology database. Am J Prev Med 1999; 17:203–206Crossref, Medline, Google Scholar
14. : Disparities in mammography use among US women aged 40–64 years, by race, ethnicity, income, and health insurance status, 1993 and 2005. Med Care 2008; 46:692–700Crossref, Medline, Google Scholar
15. : Predictors and course of vocational status, income, and quality of life in people with severe mental illness: a naturalistic study. Soc Sci Med 2007; 65:1420–1429Crossref, Medline, Google Scholar
16. : Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res 2009; 9:127Crossref, Medline, Google Scholar
17. : Using concept mapping to explore barriers and facilitators to breast cancer screening in formerly homeless women with serious mental illness. J Health Care Poor Underserved 2015; 26:908–925Crossref, Medline, Google Scholar
18. : Breast and cervical cancer screening for women with mental illness: patient and provider perspectives on improving linkages between primary care and mental health. Arch Womens Ment Health 2007; 10:189–197Crossref, Medline, Google Scholar
19. : Increased risk of perforated appendicitis in patients with schizophrenia and dementia: a population-based case-control study. Medicine 2020; 99:e18919Crossref, Medline, Google Scholar
20. : Association of secondary preventive cardiovascular treatment after myocardial infarction with mortality among patients with schizophrenia. JAMA Psychiatry 2018; 75:1234–1240Crossref, Medline, Google Scholar
21. : Reducing global disparities in cancer screening for people with mental illness. Lancet Psychiatry 2020; 7:4–6Crossref, Medline, Google Scholar
22. : Cancer care for individuals with schizophrenia. Cancer 2014; 120:323–334Crossref, Medline, Google Scholar
23. : Hepatitis C screening among Medicaid patients with schizophrenia, 2002–2012. Schizophr Bull Open 2022; 3:sgab058Crossref, Medline, Google Scholar
24. : Drug use patterns in severely mentally ill Medicare beneficiaries: impact of discontinuities in drug coverage. Health Serv Res 2008; 43:496–514Crossref, Medline, Google Scholar
25. : American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89Crossref, Medline, Google Scholar
26. : Distinguishing screening from diagnostic mammograms using Medicare claims data. Med Care 2014; 52:e44–e51Crossref, Medline, Google Scholar
27. : Can Medicare billing claims data be used to assess mammography utilization among women ages 65 and older? Med Care 2006; 44:463–470Crossref, Medline, Google Scholar
28. : Mammography among women with severe mental illness: exploring disparities through a large retrospective cohort study. Psychiatr Serv 2018; 69:48–54Link, Google Scholar
29. : Clinical care quality among Veterans Health Administration patients with mental illness following medical home implementation. Psychiatr Serv 2019; 70:816–823Link, Google Scholar
30. : Low rates of HIV testing among adults with severe mental illness receiving care in community mental health settings. Psychiatr Serv 2017; 68:443–448Link, Google Scholar
31. : Diabetes screening among underserved adults with severe mental illness who take antipsychotic medications. JAMA Intern Med 2015; 175:1977–1979Crossref, Medline, Google Scholar
32. : Racial disparities in screening mammography in the United States: a systematic review and meta-analysis. J Am Coll Radiol 2017; 14:157–165.e9Crossref, Medline, Google Scholar
33. : Breast, cervical, and colorectal cancer screening: patterns among women with Medicaid and commercial insurance. Am J Prev Med 2019; 57:394–402Crossref, Medline, Google Scholar
34. : A critical theoretical approach to cancer disparities: breast cancer and the social determinants of health. Front Public Health 2021; 9:674736Crossref, Medline, Google Scholar
35. : Social determinants of breast cancer screening in urban primary care practices: a community-engaged formative study. Womens Health Issues 2012; 22:e429–e438Crossref, Medline, Google Scholar
36. : Intersecting disadvantage: unpacking poor outcomes within early intervention in psychosis services. Early Interv Psychiatry 2019; 13:488–494Crossref, Medline, Google Scholar
37. Breast and Cervical Cancer Early Detection Program Report. Albany, New York State Department of Health, Cancer Services Program, n.d. https://www.health.ny.gov/diseases/cancer/cervical/resources/docs/2016-2017_cervical_cancer_prevention_report.pdfGoogle Scholar
38. : Integrated care: integrating general medical and behavioral health care: the New York State perspective. Psychiatr Serv 2013; 64:828–831Link, Google Scholar
39. : State mental health policy: mending Missouri’s safety net: transforming systems of care by integrating primary and behavioral health care. Psychiatr Serv 2009; 60:585–588Link, Google Scholar
40. : Gold Award: community-based program: a health care home for the “whole person” in Missouri’s community mental health centers. Missouri community mental health center health home program, Jefferson City, Missouri. Psychiatr Serv 2015; 66:e5–e8Link, Google Scholar
41. : Measurement of Health Status for People With Serious Mental Illness. Alexandria, VA, National Association of State Mental Health Program Directors, 2008Google Scholar
42. : Disparities in screening and treatment of cardiovascular diseases in patients with mental disorders across the world: systematic review and meta-analysis of 47 observational studies. Am J Psychiatry 2021; 178:793–803Link, Google Scholar
43. : Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry 2021; 8:883–891Crossref, Medline, Google Scholar
44. : The relevance of the Affordable Care Act for improving mental health care. Annu Rev Clin Psychol 2016; 12:515–542Crossref, Medline, Google Scholar
45. : There is little experience and limited data to support policy making on integrated care for dual eligibles. Health Aff 2012; 31:1176–1185Crossref, Google Scholar
46. : Breast and cervical cancer screening among Medicaid beneficiaries: the role of physician payment and managed care. Med Care Res Rev 2020; 77:34–45Crossref, Medline, Google Scholar



