Implementation of a Community-Partnered Research Suicide-Risk Management Protocol: Case Study From Community Partners in Care
Abstract
Objective:
Suicidality is common among participants in clinical trials and health services research, but approaches to suicide risk assessment and mitigation vary widely. Studies involving vulnerable populations with limited access to care raise additional ethical concerns. The authors applied a community-partnered approach to develop and implement a suicide-risk management protocol (SRMP) in a depression study in an underresourced setting in Los Angeles.
Methods:
Using a community-partnered participatory research framework, the authors designed and adapted the SRMP. Qualitative data regarding SRMP implementation included notes from SRMP development meetings and from study clinicians conducting outreach calls to study participants. Analyses included baseline and 6- and 12-month telephone survey data from 1,018 enrolled adults with moderate to severe depressive symptoms (8-item Patient Health Questionnaire score ≥10), of whom 48% were Black and 40% Latino.
Results:
Community stakeholders prioritized a robust SRMP to ensure participant safety. Features included rapid telephone outreach by study clinicians in all cases of reported recent suicidality and expedited treatment access. Using a suicidality timeframe prompt of “in the past 2 weeks,” endorsement of suicidality was common (15% at baseline, 32% cumulative). Midway through the study, the SRMP was modified to assess for present suicidality, which reduced the frequency of clinician involvement. Overall, 318 outreach calls were placed, with none requiring an emergency response. Treatment referrals were provided in 157 calls, and outreach was well received.
Conclusions:
SRMP implementation in research involving underresourced and vulnerable communities merits additional considerations. Partnering with community stakeholders can facilitate the development of acceptable and feasible SRMP procedures.
HIGHLIGHTS
Using a community-partnered participatory research framework, the authors developed and implemented a suicide-risk management protocol and used it in a depression study in an underresourced urban setting.
Community stakeholders prioritized participant safety, leading to unique protocol features such as rapid telephone outreach by study clinicians in all cases of suicidality endorsement and expedited access to mental health treatment.
Suicidality was endorsed by nearly a third of all study participants at one or more time points.
Outreach by study clinicians was labor intensive but well received.
Suicidality is common, with an estimated 12-month prevalence of 4% in U.S. adults (1), and suicide prevention is a national research priority (2). Although the literature describing interventions to address suicidality in clinical practice is growing, management of suicidality detected in research study activities, such as participant enrollment and surveys, remains understudied (3).
Both the U.S. Food and Drug Administration and the National Institute of Mental Health have issued guidelines for developing study-specific suicide-risk management protocols (SRMPs) to address suicidal ideation and behaviors arising during research study operations (4–6). Nonetheless, because of the absence of empirically supported procedures to address suicidality while conducting clinical research, many studies have excluded individuals with suicidal thoughts or behavior, citing safety concerns, potential impact on study retention and outcomes, and protection of study participants (3, 5–7). In studies in which participants with suicidality have not been excluded, SRMPs vary widely, ranging from direct outreach by a trained mental health professional in response to every statement of suicidal ideation to multistep, automated algorithms, with direct clinician evaluation and intervention only in high-risk cases (8–12).
Suicidal thoughts are common, but suicide attempts are rare, and suicide risk is difficult to predict with commonly used screening tools (1, 13). For example, although a positive screen for suicidality on the 9-item Patient Health Questionnaire (PHQ-9) predicts increased relative risk for attempted suicide, the absolute risk remains low (14, 15). Challenges in efficient and accurate screening, combined with a need to manage study costs and demands on study staff (11, 16, 17) and a concern about potential impacts of suicide interventions on study outcomes (6), may lead researchers to favor low-intervention SRMPs, particularly in large pragmatic trials.
These practical concerns are weighed against an ethical one: What obligation do clinical researchers have to ensure the safety of study participants expressing suicidality? This question is particularly salient in studies involving low-income, historically marginalized communities, which are underrepresented in clinical research. In such communities, SRMP implementation may be complicated by limited availability of formal mental health services as well as by hesitation of study participants to seek treatment because of stigma surrounding depression and suicidality (18–20). Furthermore, distrust of mental health providers and researchers persists in some communities of color because of a legacy of racism and research abuses, such as the Tuskegee Study (21), coupled with negative experiences, including involuntary hospitalization, when seeking treatment (19, 22). To our knowledge, the existing literature has not addressed standards for responsible and ethical responses to suicidality arising in research in underresourced, racial-ethnic minority communities.
Here, we describe how a community-academic partnership applied a community-partnered participatory research (CPPR) framework to design a unique SRMP for use in the Community Partners in Care (CPIC) depression study, carried out in two underresourced, predominantly Black and Latino communities in Los Angeles (23). As a variant of community-based participatory research (24), CPPR emphasizes community-academic coleadership, co-ownership, and knowledge exchange in all research phases (25, 26). We describe the subsequent implementation and adaptation of the SRMP using data from SRMP workgroup notes and study clinicians’ e-mail summaries of outreach calls to participants expressing suicidality. We assessed the burden of suicidality among research participants at baseline and at 6- and 12-month follow-ups. Finally, we discuss the implications of these findings and future directions for an ethical approach to addressing suicidality in clinical research involving underresourced communities.
Methods
Setting
CPIC was a group-level randomized comparative effectiveness trial designed to compare two depression collaborative care implementation approaches (23). Participants were enrolled from 93 participating programs, including primary care clinics, outpatient mental health clinics, substance outpatient and residential treatment programs, homeless and housing services, social services, and other community-based services (faith-based organizations, hair salons, exercise centers, and park and recreation senior centers) in two Los Angeles communities. Potential participants were screened for eligibility in program waiting rooms and at events. Study surveys were completed by telephone at baseline, 6 months, and 12 months from April 2010 through March 2012. The project was approved by the institutional review board (IRB) of RAND Corporation and by Los Angeles County and other agencies requiring separate review. All participants provided informed consent.
Participants
Study eligibility was limited to adults (ages ≥18 years) speaking English or Spanish, with at least moderate depressive symptoms indicated by an 8-item PHQ (PHQ-8) (27) score of ≥10 at screening. The analytic sample included 1,018 enrolled individuals who completed at least one survey (baseline, 6 months, or 12 months). Participant demographic characteristics have been previously described (28). Briefly, the study sample was 58% female (N=595); was 48% Black (N=487), 40% Latino (N=409), and 8% White (N=43); and had a mean±SD age of 46±13 years. Relevant social characteristics included 53% (N=536) having multiple risk factors for homelessness, 74% (N=750) meeting federal poverty criteria, 20% (N=205) working for pay, and 54% (N=545) being uninsured. Additional details on recruitment, enrollment, and outcomes can be found in previous publications (23, 28).
Community-Partnered Approach to SRMP Development and Implementation
Using a CPPR framework (25, 26), a workgroup comprising academic and community partners, including a community health advocate, a nurse from a local mental health clinic, a clinical administrator in the Los Angeles County Department of Mental Health, a substance abuse agency administrator, a public health nurse, and the director of a safety-net primary care clinic developed the CPIC SRMP. The workgroup modified the SRMP described in the study grant proposal on the basis of the Partners in Care study (29) during biweekly meetings over 4 months. The revised SRMP was reviewed at a community forum attended by >80 local stakeholders, including representatives from community groups, consumer and faith-based organizations, local health clinics, and social services agencies, and was subsequently reviewed and approved by the CPIC Executive Committee. The SRMP content and modifications are described in the Results section.
Data Sources
Participants self-reported gender and race-ethnicity at screening. Suicidality screening was conducted by telephone at baseline and at the 6- and 12-month follow-up surveys. Qualitative data included meeting notes from the SRMP development workgroup and study clinicians’ e-mails summarizing contact with study participants endorsing suicidal ideation. E-mail text was abstracted into Microsoft Excel and included participant study identification; event date; study data collection phase (i.e., screening, baseline, or 6- or 12-month survey); study clinician; assessment of imminent risk; intervention, such as referral to a clinic or emergency room or a 911 call; and narrative comments.
Suicidality Screening Measures
Screening for suicidality with the baseline survey utilized the following Mini-International Neuropsychiatric Interview (MINI) (30) item: “Over the past 2 weeks, when you felt depressed or uninterested, did you repeatedly consider hurting yourself, feel suicidal, or wish that you were dead? Did you attempt suicide or plan a suicide?” At 6 and 12 months, screening utilized the following PHQ-9 (31) item: “In the past 2 weeks, have you been bothered by thoughts that you would be better off dead or of hurting yourself in some way?” In the modified SRMP, participants responding affirmatively to the PHQ-9 suicidality item were asked the follow-up question: “Are these thoughts bothering you now?”
Statistical Analysis
A chi-square test of independence was performed to examine the relationship between demographic characteristics (race-ethnicity and gender) and cumulative prevalence of suicidal ideation. We ran these analyses with SAS, version 7.4, software.
Qualitative Analysis
Workgroup meeting notes were summarized to describe the process of SRMP development and adaptation. For analysis of outreach call notes, three study team members drafted codes based on both a priori areas of interest (e.g., “problems with access to care” and “referral provided”) and emergent themes (e.g., “knows how to get help”). After several rounds of analysis in which e-mails were independently coded and reviewed for agreement, a final codebook was developed and applied.
Results
Community-Partnered SRMP Themes
During initial workgroup meetings, several broad themes emerged that guided the SRMP development, adaptation, and implementation. Community partners emphasized the importance of ensuring participant safety over and above IRB requirements, with a high priority placed on suicide prevention. At the same time, community partners were conscious of the stigma of mental illness and of potential distrust of law enforcement involved with mental health crisis interventions. Both community and academic partners shared concerns about ensuring the feasibility of timely implementation of SRMP procedures within the constraints of study resources. Finally, the group agreed that an iterative process, based on CPPR principles, would be used during study implementation to modify the SRMP if necessary. Community feedback and subsequent modifications are summarized in Table 1.
| SRMP component | Feedback | Partnered solution |
|---|---|---|
| Initial design | ||
| Completion of outreach calls would take place within 24 hours of initial endorsement of suicidality. | A delayed response may be insufficient for emergent suicidality. | Completion of outreach calls by study clinicians took place within 30 minutes whenever possible. |
| Study participants with suicidal ideation would receive a list of referrals to local emergency rooms, community mental health clinics, and counseling centers. | Participants may face barriers in accessing care, including fear and stigma, unfamiliarity with how to navigate referral clinics, and challenges getting an expedited appointment in a busy safety-net system. | Written agreements were developed with local mental health and primary care clinics to provide facilitated referrals through a “warm handoff,” with appointments in 1–2 business days. Participants already in treatment were coached on how to discuss suicidal ideation with their current providers. |
| Low threshold for study clinicians to contact 911 or the Los Angeles County PMRT for further evaluation of suicidality. | Because of historical relationships, study communities may be distrustful of local law enforcement and fearful of the possibility of involuntary detainment or hospitalization. | PMRT or 911 emergency response would be contacted by study clinicians only if, on detailed assessment, a concern for imminent threat of self-harm emerged and no other reasonable options for ensuring study participant safety existed. |
| Midstudy modification | ||
| Direct outreach took place by a study clinician to every participant with a positive suicidality screen on the MINI (at baseline) or the PHQ-9 (on 6- and 12-month surveys). | Positive suicidality screening was very common, leading to a heavy burden of outreach calls on clinical staff. Despite frequent suicidality, no participants were assessed by study clinicians to be at imminent risk of self-harm. | Study participants screening positive on the MINI or PHQ-9 suicide item were asked an additional question (“Are these thoughts bothering you now?”) initially used by clinicians in their follow-up telephone contacts. Study clinician outreach was limited to participants who responded affirmatively to this “current suicidality” follow-up question. |
TABLE 1. Community-partnered adaptations to the suicide-risk management protocol (SRMP) for 1,018 enrolled adults with moderate to severe depressive symptomsa
SRMP Procedures
The SRMP was activated in response to suicidality endorsed on the MINI or PHQ-9 or disclosed to survey staff. After the activation, staff immediately paged the on-call study clinician (a licensed physician or psychologist) and shared the participant’s name, telephone number, preferred language, and reason for SRMP activation. Within 30 minutes, the study clinician placed an outreach call to the participant, with ongoing attempts if the initial call was unsuccessful. For Spanish speakers, translation was provided via a three-way call. At least one of six study clinicians was on call during all hours of survey operation, including evenings and weekends, over an 18-month period.
Study clinicians received guidelines for follow-up and resources for referrals. If the clinician determined that a participant was at high risk for self-harm, the clinician would facilitate a referral to an emergency department or, in case of imminent risk and as a last resort, call 911. Participants deemed at low immediate risk, and with a current outpatient provider, were coached in how to discuss suicidality with the provider; if assistance was needed, permission could be obtained for the study clinician to speak to the provider. For participants with no current provider, the study clinician would provide a referral to a partner mental health or primary care agency where the patient would be seen within 2 business days, with a “warm handoff” during which study staff would speak directly to a clinic staff member.
Suicidality Prevalence
Suicidal thoughts were common, endorsed by 32% of study participants at one or more of the three survey time points, and by 15% of participants at baseline, 24% at the 6-month follow-up, and 20% at the 12-month follow-up (Table 2). Of all participants endorsing suicidal thoughts during the study (N=329), 215 (65%) did so at a single survey time point, 89 (27%) at two time points, and 25 (8%) at all three time points. As shown in Table 3, overall prevalence of suicidal ideation was higher for men than for women (37% vs. 29%; p=0.01); no statistically significant differences were detected among different race-ethnicities.
| Positive SI by MINI and PHQ-9 | Positive current SI | |||||
|---|---|---|---|---|---|---|
| Survey | Suicidality screening tool | Total N | N | % | N | % |
| Baseline | MINIb | 980 | 144 | 15 | NA | |
| 6 months | PHQ-9c | 424 | 100 | 24 | NA | |
| 6 months (modified SRMP) | PHQ-9+current SI questionc,d | 336 | 79 | 24 | 30 | 9 |
| 12 months (modified SRMP) | PHQ-9+current SI questionc,d | 733 | 146 | 20 | 44 | 6 |
TABLE 2. Prevalence of suicidal ideation (SI) by survey and screening tool for adults with moderate to severe depressive symptomsa
| Positive SI | ||||
|---|---|---|---|---|
| Group | Total N | N | % | p |
| Overall | 1,018 | 329 | 32 | |
| Race-ethnicity | .86 | |||
| Latino | 409 | 136 | 33 | |
| Black | 487 | 153 | 31 | |
| Non-Hispanic White | 86 | 27 | 31 | |
| Other | 35 | 13 | 37 | |
| Gender | .01 | |||
| Male | 423 | 155 | 37 | |
| Female | 595 | 174 | 29 | |
TABLE 3. Cumulative prevalence of SI by race and gender for the enrolled adults with moderate to severe depressive symptomsa
SRMP Modification
As the study progressed, we noted that no participants had been deemed to be at high immediate risk of self-harm on the outreach calls, despite the high frequency of SRMP activation; some participants reported passive thoughts of death or hopelessness without intent or plan to act on those thoughts, whereas others reported current resolution of suicidal thoughts. Midway through the 6-month follow-up survey, after discussions involving community partners, research staff, and the RAND IRB, the decision was made to modify the SRMP (Table 1). Subsequently, participants screening positive for recent suicidality on the PHQ-9 (used for the 6- and 12-month surveys) were asked an additional question to assess current suicidality: “Are these thoughts bothering you now?” Outreach was triggered only when a participant answered affirmatively. Before the SRMP modification, this key question was posed by study clinicians during the outreach calls, and its incorporation into the survey limited outreach calls to assessment of ongoing suicidal ideation, reducing burden on the study staff.
SRMP Implementation
Of 1,018 participants surveyed, study clinician outreach was triggered in 318 instances across 253 unique participants during telephone surveys at three time points (Figure 1). Participants were successfully contacted in 300 instances; all of them were found to be at low immediate risk for suicide. In 157 outreach calls, participants were provided one or more referrals, which included mental health clinics (N=83), emergency room recommendations for future reference (N=80), and a suicide hotline number (N=66). Referrals were either declined or deemed unnecessary (e.g., because the participant was already in treatment) in 137 calls. Referral data were missing for six calls. Study clinicians were unable to reach a participant in 18 cases, six of whom were determined to be at low immediate risk on the basis of further discussion with the participant’s study survey administrator or outpatient clinician; the 12 remaining cases were coded as lost to follow-up.
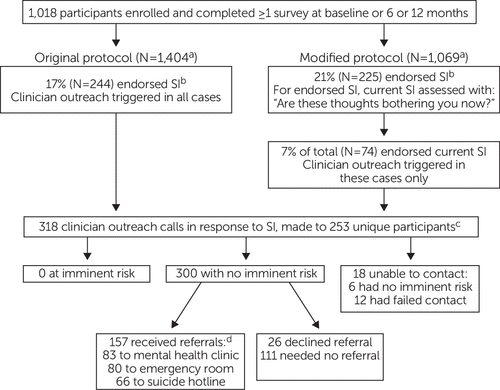
FIGURE 1. Implementation of the Community Partners in Care suicide-risk management protocol
aIncludes suicide screening surveys from multiple time points.
bSI, suicidal ideation; assessed with the Mini-International Neuropsychiatric Interview or the 9-item Patient Health Questionnaire (PHQ-9) under original protocol and the PHQ-9 under modified protocol.
cSome participants received calls at more than one time point.
dSome participants received multiple referrals.
As detailed above, the SRMP was modified midway through the study, leading to a higher threshold for study clinician outreach. Under the original protocol, 244 calls were triggered, representing 17% of telephone surveys (N=1,404). Under the modified protocol, 74 calls were triggered after 225 initial reports of suicidal ideation, representing 7% and 21% of telephone surveys (N=1,069) endorsing current and previous or current suicidal ideation, respectively. Outreach frequency did not vary by CPIC study arm (see online supplement to this article).
In the 4-year follow-up of the CPIC study, which included intensive tracking of all enrolled participants and review of death records, no known suicide deaths occurred among the study participants. One study participant was hospitalized for a nonfatal suicide attempt 3 weeks after the baseline survey, at which time he had endorsed suicidal thoughts, received a study clinician outreach call, and was found to be at low immediate risk for suicide.
Participant Responses to Study Clinicians’ Outreach
Key themes identified from study clinicians’ notes included discussion of participants’ current stressors, such as personal or family illness; housing, financial, or legal problems; trauma history; and difficulty either obtaining or remaining in mental health treatment. Participants often expressed surprise and appreciation in response to the clinicians’ outreach. Quotes from participants included the following: “This program is wonderful—it should be a national resource for communities like ours,” “I wish this project could be available to everyone in the community,” and “You’re the only person who has ever called me just to check on how I’m doing.” Some participants expressed skepticism about treatment: “What would an old person like me do talking to someone about how I feel? I mean, how could it help?” No complaints were noted about violation of privacy.
Discussion
Exclusion of people of color from clinical research has limited the widespread applicability of research findings (18, 32). Correction of this exclusion is increasingly viewed as a national priority, highlighted by a mandate that studies funded by the National Institutes of Health address inclusion of women and racial-ethnic minority groups (33). In our study, community stakeholders made clear that conducting research in Black and Latino communities with limited access to health care and a legacy of negative interactions with the academic and medical establishments requires careful consideration of how to address suicidality ethically and responsibly.
Using a CPPR framework, our community-academic partnership developed an SRMP that significantly differed from those in the published literature, grounded in community partners’ emphasis that the protocol go beyond basic risk assessment and mitigation and actively prevent any study-related suicides. First, the SRMP featured a low threshold for rapid, high-quality outreach, assessment, and brief counseling by a licensed clinician. Although the study was not designed to measure the impact of the clinician outreach, the outreach calls themselves may have served as a therapeutic intervention. Second, the SRMP included arrangements with local outpatient clinics to facilitate expedited mental health intake for individuals not currently in treatment. Given that limited access to appropriate care is a well-established barrier to depression treatment, particularly for Black and Latino individuals (18, 34, 35), these referrals may have served as an important bridge to treatment.
Outreach calls were well received but labor intensive, and it was challenging to ensure adequate staffing to respond to the high volume of positive screenings for suicidality. Thus, midway through the study, the protocol was modified to raise the threshold for study clinician outreach. Similar to findings by Corson et al. (36), only about one-third of participants endorsing the PHQ-9 screening item reported ongoing suicidality in the follow-up question (“Are these thoughts bothering you now?”). Given the poor predictive value of many suicide screening items (13), researchers may consider incorporating key follow-up questions into study surveys, as we did in the modified protocol. Our results highlight the tension around how to design an SRMP that reflects a commitment to the safety of the study community while also being feasible to implement, particularly in large trials such as this one. Academic-community partnerships must weigh these considerations when developing future SRMPs.
The CPIC study was not designed to track suicidality or SRMP implementation, leading to several limitations. First, clinicians’ e-mails summarizing outreach calls varied in detail, limiting the depth and precision of the analyses. Second, two different suicidality screening tools were used: the MINI at baseline and the PHQ-9 in subsequent surveys. Both items address thoughts of self-harm or death, but the MINI includes the word “suicide” (see Methods for full items); this difference may explain the lower endorsement when the MINI was used (15%) rather than the PHQ-9 (22%). Finally, because of the somewhat nonspecific language of the screening items, our results do not provide detailed information on forms of ideation, which may range from thoughts of nonsuicidal self-harm, to passive thoughts of death, to a specific suicide plan.
The results of our study reveal a high burden of suicidality among individuals with depression in two low-income, predominantly Black and Latino communities in Los Angeles, with 32% of the participants endorsing suicidal ideation at one or more time point. Despite researchers’ concerns about community stigma associated with depression and suicidality, the participants largely welcomed and appreciated the outreach calls by the study clinicians. Our data on SRMP implementation were included in an annual report shared with community members and stakeholders, who expressed appreciation for the efforts taken to ensure the safety of potentially vulnerable study participants. We believe that engagement of community stakeholders in SRMP development facilitated the high degree of acceptability of SRMP procedures by both study participants and the broader community.
Conclusions
This work adds to the limited literature on the development of SRMPs for clinical and services research and, to our knowledge, is the first to address the particular implications of SRMPs in underresourced communities. Further research is necessary to determine the impact of interventions related to suicidality in research studies and whether similar protocols could be implemented as part of community services in response to suicidality and beyond the context of research study operations. We argue that government agencies, funders, and investigators have an obligation to assess the feasibility and effectiveness of efforts to address suicidality emerging in research studies, particularly in those involving underresourced communities.
1 Piscopo K, Lipari RN, Cooney J, et al: Suicidal Thoughts and Behavior Among Adults: Results from the 2015 National Survey on Drug Use and Health. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2016. https://www.samhsa.gov/data/sites/default/files/NSDUH-DR-FFR3-2015/NSDUH-DR-FFR3-2015.htm. Accessed Aug 24, 2020Google Scholar
2
3 : Addressing risks to advance mental health research. JAMA Psychiatry 2013; 70:1363–1371Crossref, Medline, Google Scholar
4 Guidance for Industry: Suicidal Ideation and Behavior: Prospective Assessment of Occurrence in Clinical Trials. Silver Spring, MD, , 2012Google Scholar
5 : Ethical issues in including suicidal individuals in clinical research. IRB 2002; 24:9–14Crossref, Medline, Google Scholar
6 : Intervention research with persons at high risk for suicidality: safety and ethical considerations. J Clin Psychiatry 2001; 62(suppl 25):17–26Medline, Google Scholar
7 : Protection of human subjects in intervention research for suicidal behavior. Am J Psychiatry 2004; 161:1558–1563Link, Google Scholar
8 : Suicide risk management: development and analysis of a telephone-based approach to patient safety. Transl Behav Med 2011; 1:372–383Crossref, Medline, Google Scholar
9 : Risk for suicidal ideation in the US Air Force: an ecological perspective. J Consult Clin Psychol 2011; 79:600–612Crossref, Medline, Google Scholar
10 : Suicide risk management for the Sequenced Treatment Alternatives to Relieve Depression Study: applied NIMH guidelines. J Psychiatr Res 2004; 38:583–589Crossref, Medline, Google Scholar
11 : Current practices of suicide risk management protocols in research. Crisis 2010; 31:7–11Crossref, Medline, Google Scholar
12 : Ethical issues relevant to the assessment of suicide risk in nonclinical research settings. Crisis 2012; 33:54–59Crossref, Medline, Google Scholar
13 : Screening for suicide risk in adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2004; 140:822–835Crossref, Medline, Google Scholar
14 : Does response on the PHQ-9 Depression Questionnaire predict subsequent suicide attempt or suicide death? Psychiatr Serv 2013; 64:1195–1202Link, Google Scholar
15 : Does suicidal ideation as measured by the PHQ-9 predict suicide among VA patients? Psychiatr Serv 2016; 67:517–522Link, Google Scholar
16 : Rapid assessment procedure informed clinical ethnography (RAPICE) in pragmatic clinical trials of mental health services implementation: methods and applied case study. Adm Policy Ment Health Ment Health Serv Res 2019; 46:255–270Crossref, Medline, Google Scholar
17 : Exploring the ethical and regulatory issues in pragmatic clinical trials. Clin Trials 2015; 12:436–441Crossref, Medline, Google Scholar
18 Mental Health: Culture, Race, and Ethnicity: A Supplement to Mental Health: A Report of the Surgeon General. Rockville, MD, , 2001Google Scholar
19 : Bridging the gap: recruitment of African-American women into mental health research studies. Acad Psychiatry 2003; 27:21–28Crossref, Medline, Google Scholar
20 : Advancing suicide prevention research with rural American Indian and Alaska Native populations. Am J Public Health 2015; 105:891–899Crossref, Medline, Google Scholar
21 : Under the shadow of Tuskegee: African Americans and health care. Am J Public Health 1997; 87:1773–1778Crossref, Medline, Google Scholar
22 : A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health 2014; 104:e16–e31Crossref, Medline, Google Scholar
23 : Using a community partnered participatory research approach to implement a randomized controlled trial: planning Community Partners in Care. J Health Care Poor Underserved 2010; 21:780–795Crossref, Medline, Google Scholar
24 Wallerstein N, Duran B, Oetzel JG, (eds): Community-Based Participatory Research for Health: Advancing Social and Health Equity, 3rd ed. San Francisco, Jossey-Bass, 2018Google Scholar
25 : Begin your partnership: the process of engagement. Ethn Dis 2009; 19(suppl 6): 8–16Google Scholar
26 : Strategies for academic and clinician engagement in community-participatory partnered research. JAMA 2007; 297:407–410Crossref, Medline, Google Scholar
27 : The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009; 114:163–173Crossref, Medline, Google Scholar
28 : Community-partnered cluster-randomized comparative effectiveness trial of community engagement and planning or resources for services to address depression disparities. J Gen Intern Med 2013; 28:1268–1278Crossref, Medline, Google Scholar
29 : Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. JAMA 2000; 283:212–220Crossref, Medline, Google Scholar
30 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Medline, Google Scholar
31 : The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613Crossref, Medline, Google Scholar
32 : Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med 2015; 12:
33 NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. Pub no NOT-OD-02-001. Bethesda, MD, National Institutes of Health, 2001Google Scholar
34 : New evidence regarding racial and ethnic disparities in mental health: policy implications. Health Aff 2008; 27:393–403Crossref, Medline, Google Scholar
35 : The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry 2001; 58:55–61Crossref, Medline, Google Scholar
36 : Screening for depression and suicidality in a VA primary care setting: 2 items are better than 1 item. Am J Manag Care 2004; 10:839–845Medline, Google Scholar



