Efficacy and Safety of Pharmacotherapeutic Smoking Cessation Aids in Schizophrenia Spectrum Disorders: Subgroup Analysis of EAGLES
Abstract
Objective:
This study aimed to evaluate the efficacy and safety of varenicline, bupropion, and nicotine replacement therapy (NRT) among smokers with schizophrenia spectrum disorders in post hoc analyses of Evaluating Adverse Events in a Global Smoking Cessation Study data.
Methods:
Smokers with schizophrenia spectrum disorder (N=390) and without a psychiatric illness (control group, N=4,028) were randomly assigned to receive varenicline, bupropion, NRT patch, or placebo for 12 weeks. Outcomes included abstinence rates during treatment and follow-up, number needed to treat (NNT) for abstinence, incidence of neuropsychiatric adverse events (NPSAEs), and temporal relationship between NPSAEs and abstinence status.
Results:
Smokers with schizophrenia smoked more and had greater dependence and fewer prior trials of cessation pharmacotherapy at baseline. At each time point, smokers with schizophrenia assigned to varenicline had significantly greater odds of abstinence compared with their matched placebo group, with NNT comparable to the control group. Bupropion and NRT increased odds of abstinence; confidence intervals (CIs) included 1 for some comparisons, and NNT for smokers with schizophrenia was greater than for the control group. No treatment was associated with significantly more NPSAEs, compared with placebo, in either cohort. The estimated NPSAE rate was 5% (95% CI=3.0–7.7) for smokers with schizophrenia and 1% (95% CI=0.6–2.1) for the control group. Over one-third of NPSAEs occurred during partial or full abstinence, suggesting a multifactorial nature.
Conclusions:
For smokers with schizophrenia, varenicline led to significantly higher abstinence rates, and NNT was comparable to the control group. A significant proportion of NPSAEs occurred during early abstinence. No treatment significantly increased NPSAE prevalence.
HIGHLIGHTS
At baseline, smokers with schizophrenia spectrum disorders smoked more cigarettes per day, had greater severity of nicotine dependence, and had fewer prior trials of smoking cessation pharmacotherapy, compared with smokers without axis I psychiatric disorders.
For smokers with schizophrenia spectrum disorders, smoking cessation pharmacotherapy with varenicline was associated with higher abstinence rates compared with nicotine replacement therapy or bupropion.
The number needed to treat with varenicline was comparable for smokers with schizophrenia spectrum disorders and those without a psychiatric disorder.
None of the active smoking cessation pharmacotherapies significantly increased the prevalence of neuropsychiatric adverse events in either cohort.
Individuals with schizophrenia spectrum disorders are more likely to smoke tobacco, smoke heavily (1), and have severe dependence compared with those without psychiatric illness (2–4). Smoking rates are not decreasing for those with schizophrenia, as they are in the general population (5), and smoking-related disease contributes disproportionately to a comparative 29-year mortality gap among adults (6–9). Although quitting smoking by middle age reduces the risk of death associated with continued smoking by 90% (10), smokers with schizophrenia are less likely than those in the general population to be offered effective pharmacotherapeutic smoking cessation aids, particularly varenicline (1, 11, 12). Consistent reports of abstinence rates of less than 5% among smokers with schizophrenia who receive behavioral smoking cessation treatment alone (13–19) suggest that this group in particular needs pharmacotherapeutic cessation aids to quit smoking.
Despite evidence of the safety and efficacy of first-line pharmacotherapeutic cessation aids in this population (19, 20), clinicians report negative attitudes toward providing smoking cessation treatment for smokers with schizophrenia (21), and pharmacotherapy—particularly nonnicotine pharmacotherapy—is particularly underutilized (1, 11, 12, 22, 23). Additionally, Medicaid coverage of the most effective cessation treatments remains limited in many states, despite legislation barring state Medicaid programs from excluding cessation medications approved by the U.S. Food and Drug Administration (FDA) from coverage (24). High copays and prior authorization requirements remain common barriers to obtaining smoking cessation medication through Medicaid and Medicare plans that insure most people with schizophrenia spectrum disorders (25). Additionally, limits on access to effective smoking cessation treatment, through low rates of prescribing and financial barriers, place people with schizophrenia at increased risk of smoking-related disease and death.
The neuropsychiatric safety and efficacy trial of varenicline, bupropion, and nicotine replacement therapy (NRT) among smokers with and without psychiatric disorders (Evaluating Adverse Events in a Global Smoking Cessation Study [EAGLES]) estimated the incidence of moderate to severe neuropsychiatric adverse events (NPSAEs) during a 12-week treatment period and 12-week follow-up and assessed tobacco abstinence rates (26). NPSAE incidence and continuous abstinence rates have been reported for the schizophrenia spectrum disorders subcohort and compared with rates for the mood and anxiety disorders subcohorts (27). To address safety concerns that may drive the particular underuse of effective smoking cessation medications for smokers with schizophrenia, we undertook a post hoc analysis of weekly patterns of NPSAEs and abstinence rates in the EAGLES schizophrenia spectrum disorders subcohort compared with smokers without psychiatric disorders. Our approach included analysis of timing of NPSAEs relative to start of study medication and change in weekly 7-day point prevalence abstinence (PPA) status and analysis of end-of-treatment abstinence rates by baseline psychiatric symptom severity rating.
Methods
EAGLES was a multinational, multicenter, randomized, double-blind, placebo- and active (NRT)-controlled trial, conducted from November 30, 2011, to January 13, 2015. The primary report provides details of the design and primary outcomes (26). Study procedures and consent forms were approved by the institutional review boards at participating institutions. All participants signed informed consent.
Eligible participants were adults motivated to quit smoking, ages 18–75 years, who smoked 10 or more cigarettes per day, with expired carbon monoxide (CO) >10 parts per million (ppm) at screening. Eligible smokers with schizophrenia spectrum disorders met DSM-IV-TR (28) diagnostic criteria for current or lifetime psychotic disorders, including schizophrenia and schizoaffective disorders. Smokers with schizophrenia spectrum disorders who had other psychiatric comorbid conditions were not excluded, except for those with an alcohol or other drug use disorder active within the previous 12 months. Smokers with schizophrenia spectrum disorders made up approximately 10% of the psychiatric cohort (N=390) in EAGLES. Enrollment criteria required a score of <5 on the 7-point Clinical Global Impression–Severity (CGI-S) (29), indicating moderate severity of symptoms or less. The control cohort without psychiatric disorders (N=4,028) had no axis I diagnosis.
Random Assignment, Masking, and Study Treatment
Random assignment to receive 1 mg varenicline twice daily, 150 mg bupropion sustained-release twice daily, 21 mg NRT transdermal patch per day with taper, or placebo was done in a 1:1:1:1 ratio, with block size of eight for each diagnostic subcohort by region in a double-blind, triple-dummy, parallel-group design. Participants set a target quit date 1 week after random assignment, coinciding with the end of varenicline and bupropion up-titration and initiation of NRT. Study visits were weekly for 6 weeks, biweekly for 6 weeks, and then at weeks 13, 16, 20, and 24. Ten-minute individual smoking cessation counseling was provided at each visit (30). Telephone contacts to determine smoking status were conducted weekly between visits.
Assessments
Psychiatric diagnosis was assessed at screening with the Structured Clinical Interviews for DSM-IV-TR Axis I and II Disorders (SCID-I and SCID-II) (28, 31). Severity of cigarette dependence was assessed with the Fagerström Test for Cigarette Dependence (FTCD) (32). Abstinence was assessed weekly and defined as self-report of tobacco abstinence since the previous study assessment. Expired CO of ≤10 ppm was used to validate self-reported abstinence. CO was collected at study weeks 1–6, 8, 10, 12, 16, and 24. Participants who discontinued the study or were lost to follow-up or had missing CO data at weeks 12 or 24 were considered nonabstinent.
An NPSAE was an adverse event in one of 16 neuropsychiatric symptom categories voluntarily reported, observed, or solicited via questioning during treatment or 30-day follow-up that was new or increased in severity from baseline, irrespective of whether the adverse event was considered causally related to study medication, and that met a priori severity criteria. The following events met criteria for an NPSAE: those expected to be more common (anxiety, depression, feeling abnormal, or hostility) that were rated as severe and those rated as moderate or severe in the categories of agitation, aggression, delusions, hallucinations, homicidal ideation, mania, panic, paranoia, psychosis, suicidal ideation, suicidal behavior, or suicide.
NPSAEs were assessed at each study visit with open-ended questions, direct observation, and the semistructured Neuropsychiatric Adverse Event Interview (26, 33), which encompasses and extends beyond the psychiatric adverse events captured in the Medical Dictionary for Regulatory Activities. Positive responses on the Neuropsychiatric Adverse Event Interview were evaluated for frequency, duration, and severity to determine whether they qualified as NPSAEs. Investigators evaluated whether positive responses on the Columbia Suicide Severity Rating Scale (C-SSRS) or proxy reports from family members or others qualified as NPSAEs. Psychiatric symptoms were assessed at each study visit with the Hospital Anxiety and Depression Scale (34) and C-SSRS (35). Tobacco and nicotine use were assessed with a structured questionnaire and expired CO measurement.
Analysis
Generalized linear models (GLMs) were conducted to test the effect of treatment (varenicline, bupropion, NRT, or placebo) and cohort (schizophrenia spectrum disorders or no psychiatric disorders) on 7-day PPA and continuous abstinence rates during the treatment and follow-up periods. Observed rates of NPSAEs were reported, and GLMs were conducted to test effects of treatment, cohort, and their interaction on the NPSAE primary endpoint for 16 weeks following treatment initiation (12 weeks of treatment and 4 weeks of follow-up). All models included region, age, race, body mass index (BMI), smoking characteristics, and past cessation medication use if associated with outcome (36). For each reported NPSAE, the week of the event was plotted together with change in weekly 7-day PPA status—smoker, partial abstainer, and abstainer—by treatment assignment and cohort.
Results
Participants
The efficacy cohort consisted of all 390 and 4,028 participants enrolled in the schizophrenia and control cohorts, respectively. The safety cohort consisted of 386 (99%) and 3,984 (99%) smokers in the schizophrenia and control cohorts, respectively, who received at least one dose of study medication. The 12-week treatment and 12-week follow-up phases were completed, respectively, by 350 (90%) and 336 (86%) smokers with schizophrenia and 3,404 (85%) and 3,124 (78%) smokers without psychiatric disorders. (A CONSORT diagram is available in an online supplement to this article.)
In the schizophrenia spectrum disorders subcohort, 303 (78%) met DSM-IV-TR criteria for schizophrenia, and 87 (22%) met DSM-IV-TR criteria for schizoaffective disorder; 142 (36%) met SCID criteria for comorbid axis I disorder, including 105 (27%) with a prior alcohol or drug use disorder. Those in the schizophrenia subcohort were more likely than those in the control cohort to report suicidal ideation or behavior in their lifetime (32% versus 5%); to report greater symptoms of anxiety, depression, and aggression at baseline; and to be treated with psychotropic medications (Table 1).
| Schizophrenia spectrum disorder | No psychiatric disorder | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (N=390) | Varenicline (N=95) | Bupropion (N=98) | NRT (N=99) | Placebo (N=98) | All (N=4,028) | Varenicline (N=1,005) | Bupropion (N=1,001) | NRT (N=1,013) | Placebo (N=1,009) | |||||||||||
| Characteristic | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % |
| Demographic | ||||||||||||||||||||
| Femaleb | 140 | 36 | 34 | 36 | 33 | 34 | 38 | 38 | 35 | 36 | 2,006 | 50 | 488 | 49 | 493 | 49 | 510 | 50 | 515 | 51 |
| Age (M±SD) | 44.5±10.6 | 44.6±11.6 | 44.5±10.7 | 43.3±10.2 | 45.4±9.8 | 45.9±12.9 | 45.8±12.9 | 46.0±13.0 | 46.1±12.8 | 45.9±12.8 | ||||||||||
| Raceb | ||||||||||||||||||||
| White | 249 | 64 | 59 | 62 | 60 | 61 | 63 | 64 | 67 | 68 | 3,324 | 83 | 828 | 82 | 830 | 83 | 840 | 83 | 826 | 82 |
| Black | 126 | 32 | 33 | 35 | 36 | 37 | 31 | 31 | 26 | 27 | 514 | 13 | 139 | 14 | 118 | 12 | 130 | 13 | 127 | 13 |
| Other | 15 | 4 | 3 | 3 | 2 | 2 | 5 | 5 | 5 | 5 | 190 | 5 | 38 | 4 | 53 | 5 | 43 | 4 | 56 | 6 |
| Body mass index (kg/m2) (M±SD)b | 29.8±6.9 | 30.6±7.5 | 29.6±7.0 | 29.3±6.6 | 29.5±6.6 | 27.6±6.1 | 27.4±6.0 | 27.6±6.2 | 27.8±6.3 | 27.8±6.0 | ||||||||||
| Smoking | ||||||||||||||||||||
| FTCD score (M±SD)b,c | 6.9±1.8 | 6.8±1.9 | 6.9±1.7 | 6.8±2.0 | 7.0±1.7 | 5.5±2.0 | 5.5±2.0 | 5.5±2.0 | 5.6±2.0 | 5.5±2.0 | ||||||||||
| Cigarettes smoked per day in past month (M±SD)b | 23.2±10.7 | 22.5±9.4 | 22.2±7.4 | 23.9±14.9 | 24.3±9.5 | 20.7±8.0 | 20.7±8.3 | 20.7±7.9 | 20.8±8.2 | 20.5±7.9 | ||||||||||
| Previous quit attempts (M±SD) | 2.4±4.0 | 2.2±3.2 | 1.9±2.0 | 2.2±2.8 | 3.3±6.4 | 3.2±9.7 | 3.2±13.7 | 3.3±10.2 | 3.2±5.1 | 3.1±7.4 | ||||||||||
| Prior cessation aid trials | ||||||||||||||||||||
| Vareniclineb | 31 | 8 | 9 | 9 | 8 | 8 | 8 | 8 | 6 | 6 | 578 | 14 | 132 | 13 | 148 | 15 | 159 | 16 | 139 | 14 |
| Bupropionb,d | 14 | 4 | 3 | 3 | 6 | 6 | 2 | 2 | 3 | 3 | 373 | 9 | 95 | 9 | 93 | 9 | 94 | 9 | 91 | 9 |
| NRTb,e | 59 | 15 | 17 | 18 | 13 | 13 | 12 | 12 | 17 | 17 | 998 | 25 | 229 | 23 | 260 | 26 | 258 | 25 | 251 | 25 |
| Psychiatric | ||||||||||||||||||||
| Past alcohol use disorder | 50 | 13 | 14 | 15 | 11 | 11 | 9 | 9 | 16 | 16 | 6 | <1 | 2 | <1 | 2 | <1 | 0 | 0 | 2 | <1 |
| Past drug abuse disorderf | 90 | 23 | 24 | 25 | 21 | 21 | 18 | 18 | 27 | 28 | 5 | <1 | 2 | <1 | 1 | <1 | 0 | 0 | 2 | <1 |
| Suicidal ideationb,g | 111 | 29 | 25 | 26 | 28 | 29 | 31 | 31 | 27 | 28 | 190 | 5 | 48 | 5 | 43 | 4 | 50 | 5 | 49 | 5 |
| Suicidal behaviorb,g | 70 | 18 | 15 | 16 | 19 | 20 | 22 | 22 | 14 | 15 | 28 | <1 | 6 | <1 | 9 | <1 | 7 | <1 | 6 | <1 |
| Suicidal ideation and/or behaviorb,g | 122 | 32 | 30 | 32 | 30 | 31 | 33 | 33 | 29 | 30 | 194 | 5 | 49 | 5 | 44 | 4 | 52 | 5 | 49 | 5 |
| HADS score (M±SD)h | ||||||||||||||||||||
| Anxiety subscaleb | 5.1±4.1 | 4.6±4.0 | 5.4±4.2 | 5.5±4.5 | 5.0±3.8 | 2.8±2.7 | 2.8±2.8 | 2.7±2.7 | 2.7±2.6 | 2.9±2.8 | ||||||||||
| Depression subscaleb | 3.9±3.2 | 4.0±3.6 | 4.2±2.8 | 3.8±3.3 | 3.8±3.0 | 1.5±2.1 | 1.5±2.1 | 1.4±2.0 | 1.5±2.0 | 1.6±2.1 | ||||||||||
| BPAQ score (M±SD)b,i | 62.7±20.1 | 61.7±19.8 | 63.4±20.3 | 63.8±20.7 | 61.6±19.7 | 52.2±15.4 | 52.4±15.5 | 51.9±15.3 | 52.2±15.6 | 52.3±15.4 | ||||||||||
| Psychotropic medicationb | ||||||||||||||||||||
| Antipsychotic | 364 | 94 | 92 | 97 | 89 | 93 | 92 | 93 | 91 | 95 | 13 | <1 | 2 | <1 | 2 | <1 | 2 | <1 | 7 | <1 |
| First generation | 93 | 24 | 22 | 23 | 29 | 30 | 18 | 18 | 24 | 25 | 5 | <1 | 1 | <1 | 0 | 0 | 2 | <1 | 2 | <1 |
| Second generation | 340 | 88 | 88 | 93 | 77 | 80 | 90 | 91 | 85 | 89 | 7 | <1 | 1 | <1 | 2 | <1 | 0 | 0 | 4 | <1 |
| Clozapine | 31 | 8 | 5 | 5 | 10 | 10 | 10 | 10 | 6 | 6 | 1 | <1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | <1 |
| Antidepressant | 118 | 31 | 31 | 33 | 27 | 28 | 36 | 36 | 24 | 25 | 105 | 3 | 22 | 2 | 21 | 2 | 26 | 3 | 36 | 4 |
| Anxiolytic, hypnotic | 66 | 17 | 18 | 19 | 16 | 17 | 21 | 21 | 11 | 11 | 225 | 6 | 53 | 5 | 50 | 5 | 61 | 6 | 61 | 6 |
| Mood stabilizer | 15 | 4 | 4 | 4 | 3 | 3 | 6 | 6 | 2 | 2 | 20 | <1 | 6 | <1 | 1 | <1 | 3 | <1 | 10 | 1 |
TABLE 1. Baseline characteristics of study sample, by cohort and treatment groupa
Smokers with schizophrenia smoked more cigarettes per day than did smokers in the control group (23.2 versus 20.7) and had greater severity of cigarette dependence (FTCD total score, 6.9 versus 5.5) (Table 1). Smokers with schizophrenia were not significantly less likely than those in the control group to have made a smoking cessation attempt, 72% (N=281 of 390) of smokers in the schizophrenia subcohort reported a mean of 2.4 prior serious quit attempts, and 82% (N=3,284 of 4,028) of smokers in the control group reported a mean of 3.2 quit attempts. However, smokers with schizophrenia were significantly less likely than smokers in the control group to report prior trials of smoking cessation treatment: varenicline (8% versus 14%), bupropion (4% versus 9%), and NRT (15% versus 25%).
Tobacco-Smoking Abstinence
Seven-day PPA rates at end of treatment and follow-up.
Weekly observed 7-day PPA rates by treatment are shown in Figure 1A. Odds of 7-day end-of-treatment PPA among smokers with schizophrenia were sixfold higher with varenicline, compared with placebo, and more than twofold higher with varenicline, compared with bupropion or NRT. Odds of 7-day end-of-treatment PPA among smokers with schizophrenia were more than twofold higher with bupropion and NRT, compared with placebo (see treatment comparison figure in online supplement). Number needed to treat (NNT) among smokers with schizophrenia and among those in the control groups for 7-day end-of-treatment PPA was, respectively, five and four for varenicline, 17 and eight for bupropion, and 12 and eight for NRT (Figure 1B).
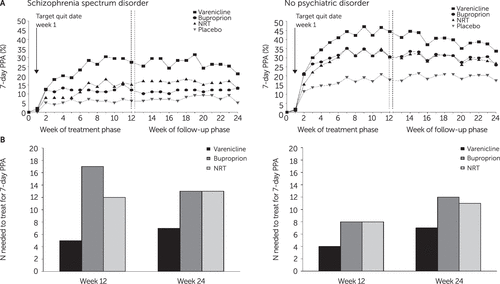
FIGURE 1. Seven-day point prevalence abstinence (PPA) rates and number needed to treat among smokers with schizophrenia and smokers without a psychiatric disordera
aA: observed 7-day PPA rates at each study visit. B: number needed to treat for 7-day PPA at end of treatment and end of follow-up. NRT, nicotine replacement therapy (transdermal nicotine patch).
At week 24, odds ratios (ORs) of 7-day PPA for active treatments versus placebo among smokers with schizophrenia ranged from 2.8 for NRT to 5.2 for varenicline (see treatment comparison figure in online supplement). In the control group, ORs for 7-day PPA for all active treatments were superior to placebo and were superior for varenicline compared with bupropion and NRT. The relative efficacy of active treatments was similar across cohorts, and abstinence rates were higher among those without psychiatric disorders (Figure 1A). NNT among smokers with schizophrenia and smokers in the control group for 7-day PPA at week 24 were, respectively, seven and seven for varenicline, 13 and 12 for bupropion, and 13 and 11 for NRT (Figure 1B).
Weekly observed and estimated continuous abstinence rates for weeks 9–12 were estimated in a model that included treatment, cohort, region, treatment × cohort, FTCD score, cigarettes per day in the past month, race, age, years smoked, and BMI (see online supplement for results). NNT among smokers with schizophrenia and among smokers in the control group for continuous abstinence for weeks 9–12 were, respectively, six and five for varenicline, 14 and nine for bupropion, and 12 and eight for NRT.
The 7-day end-of-treatment PPA rates by baseline CGI-S symptom severity category was calculated (see table in online supplement). The abstinence rate was highest among the few participants with the lowest possible baseline symptom severity; however, increasing baseline psychiatric symptom burden was not shown to be related to lower abstinence rates at end of treatment.
Neuropsychiatric safety during 12 weeks of treatment and 4 weeks of follow-up.
NPSAE rates were not significantly higher with any active treatment, compared with placebo, overall or in either cohort, and no significant treatment × diagnosis interactions were noted (Figure 2). The estimated NPSAE rate was 5% (95% CI=3.0–7.7) for smokers with schizophrenia and 1% (95% CI=0.6–2.1) for the control group. The point estimates for varenicline and NRT were negative among smokers with schizophrenia. Significant effects were noted for diagnostic cohort, region, and race. Smokers with schizophrenia were more likely than smokers in the control group to experience an NPSAE. Observed NPSAE rates among smokers with schizophrenia were as follows: varenicline, 6%; bupropion, 6%; NRT, 5%; placebo, 6% (see table in online supplement).
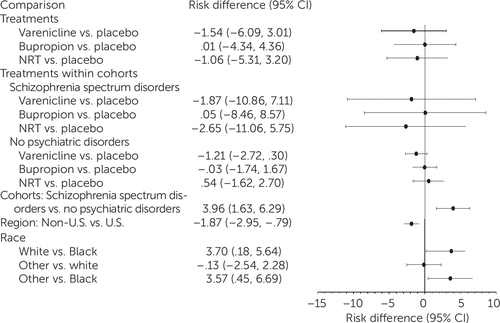
FIGURE 2. Risk differences for neuropsychiatric adverse events with varenicline, bupropion, nicotine replacement therapy (NRT), and placeboa
aPeriod for ascertainment of neuropsychiatric adverse events was during 12 weeks of treatment and ≤30 days after last dose. Model terms included treatment group (varenicline, bupropion, NRT, and placebo), cohort (schizophrenia spectrum disorders and no psychiatric disorders), treatment × cohort interaction, region (United states or non–United States), and race (white, Black, and other).
There were no observed effects of treatment on suicidal ideation or behavior, serious adverse events, or adverse events resulting in permanent discontinuations of treatment or leading to an intervention. For smokers with schizophrenia, severe NPSAEs in the primary endpoint that constituted a serious adverse event or led to treatment discontinuation or an intervention occurred as follows: varenicline, 2%; bupropion, 1%; NRT, 2%; and placebo, 2%. Serious adverse events were observed for less than 1% of both cohorts (see table in online supplement). Among smokers with schizophrenia, 22% reported an adverse event in the Medical Dictionary for Regulatory Activities’ psychiatric disorders category of mild, moderate, or severe intensity; the proportion in the control group was 30%.
In an exploratory analysis of the temporal relationship between onset of an NPSAE and 7-day PPA status by treatment assignment and cohort, eight of 23 (35%) NPSAEs among smokers with schizophrenia and 40 of 84 (48%) NPSAEs among smokers in the control group were reported at a study visit when the participant had been partially or fully abstinent in the prior week (see figure in online supplement).
Discussion
This analysis provides robust evidence for efficacy of first-line FDA-approved smoking cessation medications—particularly varenicline—among smokers with schizophrenia spectrum disorders and those without psychiatric disorders. The NNT to obtain end-of-treatment abstinence were 5 and 4, respectively, with no clear relationship in either cohort between baseline psychiatric symptom burden and attainment of abstinence or between the occurrence of NPSAEs and treatment. In both cohorts, odds of 7-day end-of-treatment PPA were higher with varenicline than with bupropion, NRT, or placebo and higher with NRT than with placebo. Prior trials have shown efficacy of varenicline (37), bupropion (14, 15), and bupropion combined with NRT for smoking cessation among smokers with schizophrenia (16, 17). EAGLES is the first trial to report efficacy of NRT versus placebo for smoking cessation among smokers with schizophrenia.
Because of the greater severity of nicotine dependence and psychiatric symptom burden among smokers with schizophrenia, these participants would be expected to be less likely to quit smoking, compared with smokers without an axis I psychiatric illness. Of note, we found no clear relationship among smokers with schizophrenia between abstinence rates and psychiatric symptom severity, and the NNT for varenicline among smokers with schizophrenia and those without psychiatric disorders was essentially equivalent (5 versus 4). Although the confidence intervals overlapped, point estimates for odds of abstinence with varenicline were nearly twice as high among smokers with schizophrenia as among smokers in the group without psychiatric disorders, which was likely attributable to very low abstinence rates for smokers with schizophrenia treated with placebo, which is consistent with prior reports of 4%−5% abstinence rates with behavioral treatment alone (14–16, 19, 37). Such low success rates indicate that it is critical for smokers with schizophrenia to have access to pharmacotherapeutic cessation aids if they are to be successful in their efforts to quit smoking. Varenicline is underutilized for smokers with psychotic illness (11, 12), and in this trial, smokers with schizophrenia reported significantly fewer prior treatment trials with varenicline, bupropion, or NRT, compared with smokers without psychiatric disorders—without significantly fewer prior cessation attempts.
It is not surprising that smokers in the schizophrenia cohort were more likely than those in the control group to experience an NPSAE during the treatment and follow-up periods, given their greater psychiatric symptom burden; higher ratings of anxiety, depression, aggression, and nicotine dependence severity; and more prior suicidal ideation and behavior. The baseline NPSAE rate for smokers with schizophrenia outside the context of a smoking cessation attempt was unknown. For smokers with schizophrenia, NPSAEs appeared to be multifactorial and sporadic: they did not cluster either within any particular symptom domain or shortly after study medication was initiated or the quit date; they occurred independently of treatment assignment; they rarely led to permanent discontinuation of smoking cessation medication; and approximately one-third were reported during study weeks with partial or complete tobacco abstinence. Serious adverse events were observed for less than 0.5% of smokers in the schizophrenia subcohort and for 0.2% of those in the cohort without psychiatric disorders. These findings are worth weighing against the well-established metric that half of smokers who do not quit will die prematurely from a smoking-related illness.
It is now considered a standard of care to offer effective smoking cessation pharmacotherapy to all smokers at every clinical visit (38), even for smokers who report that they may not be ready to make a cessation attempt. In considering the risk-benefit ratio of providing smoking cessation treatment to smokers with schizophrenia, several points are worth considering. It is increasingly recognized that smoking cessation itself does not significantly exacerbate the symptoms or course of mental illness (39, 40) and that active treatments that significantly improve abstinence rates among smokers with schizophrenia do not exacerbate psychiatric symptoms (19, 41). In EAGLES, smokers with schizophrenia treated with placebo and behavioral support were as likely as those treated with varenicline, bupropion, or NRT to experience a moderate to severe NPSAE but were far less likely to attain abstinence. The life expectancy for people with schizophrenia is approximately 29 years shorter than for those without psychiatric illness, and tobacco smoking is the single largest cause of this disparity in life expectancy (8, 42, 43), whereas smoking cessation effectively mitigates this risk (10, 44, 45). Because tobacco smoking is associated with increased hepatic clearance of many psychotropic drugs, particularly those metabolized by cytochromes P450 1A2 and 2E1, it is recommended that clinicians monitor patients who reduce or quit smoking for evidence of reduced clearance of psychotropic medications metabolized by these enzymes and consider dose adjustment accordingly (46–48).
The study had several limitations. Although 27% of smokers with schizophrenia had a prior alcohol or drug use disorder, smokers with an active alcohol or drug use disorder other than nicotine were excluded, so results cannot be expected to generalize to those with active substance use. Likewise, although participants were symptomatic at baseline, enrollment criteria required that psychiatric symptoms be stable, and 95% of smokers with schizophrenia were taking psychotropic medications. Thus, results cannot be expected to generalize to unstable or untreated smokers with schizophrenia. Although increasingly considered standard clinical care, dual NRT (NRT patch plus NRT gum, lozenge, nasal spray, or inhaler) was not tested. Future research is needed to test the effects of dual NRT for smokers with schizophrenia and to compare efficacy and tolerability of dual NRT with those of varenicline and bupropion. The behavioral component of the intervention was brief; trials of pharmacotherapy plus more intensive behavioral treatment have shown higher abstinence rates among smokers with schizophrenia (49). Further research is needed to determine whether more intensive behavioral treatment improves efficacy of pharmacotherapy for nicotine dependence among smokers with schizophrenia. We reported 24 weeks of efficacy data and 16 weeks of safety data, although clinicians and smokers will be interested in longer-term outcomes (49, 50).
Conclusions
Tobacco smokers, particularly smokers with schizophrenia, need help to quit. This study has provided robust evidence for efficacy and tolerability for smoking cessation treatments, particularly varenicline. These data, together with strong evidence that smoking cessation does not exacerbate mental illness (19, 39) and strong consistent evidence for low abstinence rates among smokers with schizophrenia with behavioral treatment alone (19, 22) and for the benefit of smoking cessation on premature mortality (10, 44), should spur lowering of barriers at the policy and practitioner levels to greater utilization of the most effective pharmacotherapeutic cessation aids for smokers with schizophrenia as a standard of care.
1 : The Stolen Years: The Mental Health and Smoking Action Report 2016. London, Action on Smoking and Health, 2016Google Scholar
2 : A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 2005; 76:135–157Crossref, Medline, Google Scholar
3 : Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend 2005; 80:259–265Crossref, Medline, Google Scholar
4 : Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob Res 2010; 12:855–859Crossref, Medline, Google Scholar
5 : Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA 2014; 311:172–182Crossref, Medline, Google Scholar
6 : Increasing global burden of cardiovascular disease in general populations and patients with schizophrenia. J Clin Psychiatry 2007; 68(suppl 4):4–7Crossref, Medline, Google Scholar
7 : A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007; 64:1123–1131Crossref, Medline, Google Scholar
8 : Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015; 72:1172–1181Crossref, Medline, Google Scholar
9 : A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res 2005; 80:45–53Crossref, Medline, Google Scholar
10 : 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013; 368:341–350Crossref, Medline, Google Scholar
11 : Use of varenicline for smoking cessation treatment in UK primary care: an association rule mining analysis. BMC Public Health 2014; 14:1024Crossref, Medline, Google Scholar
12 : Prescribing prevalence, effectiveness, and mental health safety of smoking cessation medicines in patients with mental disorders. Nicotine Tob Res 2020; 22:48–57Crossref, Medline, Google Scholar
13 : Effects of sustained-release bupropion and supportive group therapy on cigarette consumption in patients with schizophrenia. Am J Psychiatry 2001; 158:635–637Link, Google Scholar
14 : A placebo-controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry 2002; 52:53–61Crossref, Medline, Google Scholar
15 : A double-blind placebo-controlled trial of bupropion sustained-release for smoking cessation in schizophrenia. J Clin Psychopharmacol 2005; 25:218–225Crossref, Medline, Google Scholar
16 : A 12-week double-blind, placebo-controlled study of bupropion SR added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol 2007; 27:380–386Crossref, Medline, Google Scholar
17 : A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biol Psychiatry 2008; 63:1092–1096Crossref, Medline, Google Scholar
18 : Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study. Schizophr Res 2011; 129:94–95Crossref, Medline, Google Scholar
19 : Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev 2013; 2:
20 : Smoking cessation and reduction in people with chronic mental illness. BMJ 2015; 351:h4065Crossref, Medline, Google Scholar
21 : A mixed-method systematic review and meta-analysis of mental health professionals’ attitudes toward smoking and smoking cessation among people with mental illnesses. Addiction 2016; 111:1536–1553Crossref, Medline, Google Scholar
22 : Pharmacological and behavioural interventions to promote smoking cessation in adults with schizophrenia and bipolar disorders: a systematic review and meta-analysis of randomised trials. BMJ Open 2019; 9:
23 : Does varenicline worsen psychiatric symptoms in patients with schizophrenia or schizoaffective disorder? A review of published studies. J Clin Psychiatry 2012; 73:e1039–e1047Crossref, Medline, Google Scholar
24 Patient Protection and Affordable Care Act; in Health-Related Portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC, Office of the Legislative Counsel for the Use of the US House of Representatives, 2010Google Scholar
25 : State Medicaid coverage for tobacco cessation treatments and barriers to accessing treatments—United States, 2015–2017. MMWR Morb Mortal Wkly Rep 2018; 67:390–395Crossref, Medline, Google Scholar
26 : Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016; 387:2507–2520Crossref, Medline, Google Scholar
27 : Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with psychotic, anxiety, and mood disorders in the EAGLES trial. J Clin Psychopharmacol 2019; 39:108–116Crossref, Medline, Google Scholar
28 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. New York, New York State Psychiatric Institute, Biometrics Research, 2002Google Scholar
29 : ECDEU Assessment Manual for Psychopharmacology. Rockville, MD, US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, 1976Google Scholar
30 Treating Tobacco Use and Dependence: 2008 Update, Rockville, MD, US Department of Health and Human Services, 2008Google Scholar
31 : Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). Washington, DC, American Psychiatric Press, Inc, 1997Google Scholar
32 : Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine Tob Res 2012; 14:75–78Crossref, Medline, Google Scholar
33 : Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Ann Intern Med 2013; 159:390–400Crossref, Medline, Google Scholar
34 : The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67:361–370Crossref, Medline, Google Scholar
35 : The Columbia Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011; 168:1266–1277Link, Google Scholar
36 : Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in EAGLES. Addiction 2018; 113:1507–1516Crossref, Medline, Google Scholar
37 : A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2012; 73:654–660Crossref, Medline, Google Scholar
38 : 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2018; 72:3332–3365Crossref, Medline, Google Scholar
39 : Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014; 348:g1151Crossref, Medline, Google Scholar
40 : The relationship between smoking cessation and binge drinking, depression, and anxiety symptoms among smokers with serious mental illness. Drug Alcohol Depend 2019; 194:128–135Crossref, Medline, Google Scholar
41 : Improved depressive symptoms in adults with schizophrenia during a smoking cessation attempt with varenicline and behavioral therapy. J Dual Diagn 2017; 13:168–178Crossref, Medline, Google Scholar
42 : Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One 2011; 6:
43 : Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care 2011; 49:599–604Crossref, Medline, Google Scholar
44 : The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013; 381:133–141Crossref, Medline, Google Scholar
45 : Weight gain and 10-year cardiovascular risk with sustained tobacco abstinence in smokers with serious mental illness: a subgroup analysis of a randomized trial. J Clin Psychiatry 2016; 77:e320–e326Crossref, Medline, Google Scholar
46 : Treatment of tobacco use disorders in smokers with serious mental illness: toward clinical best practices. Harv Rev Psychiatry 2015; 23:90–98Crossref, Medline, Google Scholar
47 : Drug interactions with tobacco smoking: an update. Clin Pharmacokinet 1999; 36:425–438Crossref, Medline, Google Scholar
48 : A distribution study of CYP1A2 phenotypes among smokers and non-smokers in a cohort of healthy Caucasian volunteers. Eur J Clin Pharmacol 1998; 53:361–367Crossref, Medline, Google Scholar
49 : Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA 2014; 311:145–154Crossref, Medline, Google Scholar
50 : Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res 2017; 183:124–129Crossref, Medline, Google Scholar



