Timeliness of Autism Spectrum Disorder Diagnosis and Use of Services Among U.S. Elementary School–Aged Children
Abstract
Objective:
This study assessed the relationship of timeliness of autism spectrum disorder (ASD) diagnosis with current use of ASD-related services in a nationally representative sample of U.S. children.
Methods:
The Centers for Disease Control’s (CDC’s) Survey of Pathways to Diagnosis and Services was used to assess experiences of 722 children ages six to 11 with ASD. Bivariate and multivariate analyses were used to explore associations between age at ASD diagnosis and delay in ASD diagnosis and use of health services. Health services included current use of behavioral intervention (BI) therapy, school-based therapy, complementary and alternative medicine (CAM), and psychotropic medications.
Results:
Mean age at ASD diagnosis was 4.4 years, and mean diagnostic delay was 2.2 years. In adjusted analysis, older age at diagnosis (≥4 versus <4) was associated with lower likelihood of current BI or school-based therapy use and higher likelihood of current psychotropic medication use. Analyses that treated age at diagnosis as a continuous variable found that likelihood of current psychotropic medication use increased with older age at diagnosis. A delay of two or more years between parents’ first discussion of concerns with a provider and ASD diagnosis was associated with higher likelihood of current CAM use. Likelihood of current CAM use increased as delay in diagnosis became longer.
Conclusions:
Both older age at diagnosis and longer delay in diagnosis were associated with different health services utilization patterns among younger children with ASD. Prompt and early diagnosis may be associated with increased use of evidence-based therapies for ASD.
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by atypical social communication and interaction coupled with behaviors and interests that are restricted, repetitive, or both (1). ASD is common, affecting one in 68 U.S. children (2). Signs of ASD usually develop in the first two years of life (3,4). Although diagnosis usually can be made by age two, the average age at diagnosis in the United States is over four years old (2). Delay between emergence of signs and ASD diagnosis is noteworthy because early intensive treatments may have long-term benefits for child functioning and family life (5–8).
Primary care providers (PCPs) are essential to early ASD detection, given that they are in frequent contact with parents during a child’s first years. Nonetheless, some PCPs give false reassurance (9) or fail to direct families to diagnostic resources when valid parental concerns exist (10,11). Although major pediatric organizations recommend routine ASD screening (12,13), only around half of PCPs screen for ASD (14–16). Even when screening occurs, delays between initial conversations between parents and providers about possible signs of ASD and ASD diagnosis are common. Delays may be related to lack of family knowledge about ASD and the health care system, disability stigma, difficulties in understanding how the health and education systems communicate or authorize treatment, long waiting periods for evaluations, and geographic or transportation barriers (15,17,18).
When concerns about ASD exist, a child can be referred for treatment services. Some services target core ASD features, for example, by promoting social skills and reducing inflexible behaviors. Other services address ASD comorbidities, such as attention problems and anxiety (19). ASD-related service use varies by type (for example, occupational therapy and prescribed medication) and amount (for example, hours per week and medication dose) (20). Although no combination of therapy for ASD in early childhood is considered best, behavioral intervention (BI) therapy directed at core ASD symptoms has the strongest evidence of effectiveness (6–8). Other therapies—such as sensory integration therapy, complementary and alternative medicine (CAM), and psychopharmacological treatments for ASD—are more controversial (8,21,22).
Service use may correspond with timeliness of ASD diagnosis. For instance, families who experience long ASD diagnostic delays may be more likely to use CAM, especially CAM that is easily purchased, for example, nutritional supplements. Families may experience delays in diagnosis because of misdiagnosis of another condition, such as attention-deficit hyperactivity disorder, and, therefore, may use therapy or pharmacological treatments for the other condition (23). Timeliness of diagnosis may affect families’ use of government-funded services that depend on a child’s age, for example, early intervention services provided under Part C of the Individuals With Disabilities Education Act (24). As pediatric organizations press for early ASD identification (12), it is important to determine whether timely diagnosis is associated with subsequent ASD-related service use. Thus this study aimed to assess the relationship between ASD diagnostic age and delay in ASD diagnosis with current use of health services in a nationally representative sample.
Methods
Data Source and Study Sample
Data were drawn from the Centers for Disease Control’s 2011 Survey of Pathways to Diagnosis and Services (“Pathways”). Pathways was a follow-back to the 2009–2010 National Survey of Children With Special Health Care Needs (NS-CSHCN) (25), a nationally representative, parent-reported telephone survey of children identified as having special health care needs according to the CSHCN Screener (26,27). NS-CSHCN’s response rate was 25.5% (28). Parents whose children had ASD, an intellectual disability, or a developmental delay in the NS-CSHCN and were between the ages of six and 17 in 2011 were recontacted to participate in Pathways. Pathways had a telephone and a written component, but this analysis concerned only the telephone component; 71% of eligible families were successfully recontacted, and 87% of those agreed to participate in Pathways (N=4,032) (29). Methodology of NS-CSHCN and Pathways has been described and is available on the National Center for Health Statistics’ Web site (26,28,29).
Using Pathways, we assessed experiences of elementary school–aged children (ages six to 11) with current ASD. Children with an intellectual disability or a developmental delay but not ASD (N=2,098) and children with past but not current ASD, developmental delay, or intellectual disability (N=514) were excluded. Children ages 12 or older (N=698) were excluded because there are fewer ASD treatment guidelines for this group and because of concerns about recall bias (for example, parents of adolescents with ASD may not remember the child’s diagnosis age). The exclusion criteria resulted in a final sample of 722 children. The survey was approved by the National Center for Health Statistics’ Institutional Review Board.
Variables
This study used two measures of timely ASD diagnosis. The first (age at ASD diagnosis) was based on responses to the question, “How old was your child when you were first told [s/he] had autism or autism spectrum disorder [by a healthcare provider]?” The second measure (delay in ASD diagnosis) was calculated as the difference between the child’s age when a parent “first talked with a doctor or health care provider about [developmental] concerns” and the child’s age when he or she received an ASD diagnosis. Because Pathways recorded age in months up to 36 months and in years thereafter, we standardized values for age at ASD diagnosis and delay in ASD diagnosis by rounding down to the highest whole year of age (for example, ages 0–11 months were counted as zero years old, ages 12–23 months as one year old, and so on). We rounded age for three reasons: first, most parents of children ages 36 months or older report a child’s age in years rather than months—for example, parents would report a child who is three years and ten months old as age three, not age four. Second, rounding kept age measurements uniform across ages, which is critical because for many children, the interval between first conversation and diagnosis spanned the 36-month time point. Finally, rounding accounted for the difficulty parents have in remembering the exact timing of events that occurred in prior years.
Analyses treated age at ASD diagnosis and delay in ASD diagnosis as both dichotomous and continuous variables. In dichotomous analyses, older age at ASD diagnosis was defined as ages four and older, on the basis of the mean diagnosis age of the sample (4.4 years). Also, clinically, it would be possible to diagnose most children with ASD by age four (2). Similarly, we defined longer delay in ASD diagnosis as a delay of two more years between the date of the first conversation between parents and providers about possible signs of ASD and diagnosis. We dichotomized delay at two years based on the mean delay for the sample (2.2 years). Also, clinically, a delay of two or more years would be a long delay regardless of when parents initially expressed concerns because in most cases early signs of ASD are present by age two (30–32). In analyses in which age or delay was treated as continuous, the variable was considered in whole years only.
Child and family factors previously associated with age at ASD diagnosis and utilization of ASD-related health services served as covariates (33,34). Factors included child age, gender, race-ethnicity, health insurance type, functional limitations status, household income relative to federal poverty level (FPL), parent education, census region, and family structure. FPL was defined in 2011 as $22,350 per family of four (35). Insurance was categorized as “any private insurance” or “public only or uninsured,” given that many children had both private and public insurance, and only 16 were uninsured. Functional limitations status, a sensitive indicator of elevated health services use, was used as a severity marker (36) and was defined as an affirmative response to the CSHCN Screener functional limitations items (“Child is limited in any way in his/her ability to do things most children of the same age can do” because of “any medical, behavioral, or other health condition that has lasted or is expected to last 12 months or longer”) (27).
We studied four measures of ASD-related health services use; each has previously varied among children with ASD (34,37,38) or related conditions (39). Psychotropic medication use was identified if a child was taking any psychotropic medication “to meet his/her developmental needs” currently on a regular basis, including stimulants, antidepressants, anxiolytics, mood stabilizers, antiseizure medications, antipsychotics, and sleep medications. BI use was defined as a child’s use of “behavioral intervention or modification services to meet his/her developmental needs” at least once per week currently on a regular basis. CAM use was defined as a child’s use of “any type of alternative health care or treatment to meet his/her developmental needs” currently on a regular basis. School-based therapy use was defined as using social skills training or occupational, physical, or speech and language therapy at school currently on a regular basis.
Statistical Analysis
Descriptive statistics assessing the sociodemographic characteristics of the sample were computed. Chi-square tests were used to compare proportions of children receiving the four ASD health services (psychotropic medications, BI, CAM, and school-based therapy) by sociodemographic characteristic.
To examine primary outcomes, chi-square tests were computed comparing proportions of children receiving each of the health services by age at ASD diagnosis (≥4 years versus <4 years) and delay in ASD diagnosis (≥2 years versus <2 years). Logistic regression models were fit to examine unadjusted and adjusted associations between diagnostic age and delay in ASD diagnosis and use of the four health services, controlling for all child and family covariates. Logit models were fit to examine the association between continuous variables for ASD diagnosis age and delay in ASD diagnosis and use of the four health services, controlling for the same covariates. Sensitivity analyses tested whether the interaction between diagnostic age and delay in ASD diagnosis modified associations with health services use. Analyses additionally examined whether the relationship between ASD diagnosis age or delay in ASD diagnosis and services use was modified by length of ASD diagnosis (difference between age at ASD diagnosis and age when surveyed), functional limitations status, or comorbid intellectual disability diagnosis. Analyses were performed in Stata 13.1, using survey weights to account for Pathways’ complex sampling design.
Results
Of 722 children meeting inclusion criteria, mean child age was 8.9 years. Most children (73%) were non-Hispanic white, privately insured (69%), and lived in households with incomes above 200% FPL (65%); 63% had functional limitations (Table 1). On average, parents first discussed developmental concerns with a provider when the child was 2.1 years old (Figure 1). Mean age at ASD diagnosis was 4.4 years, leading to a mean diagnostic delay of 2.2 years. School-based therapy was the most frequently used health service (79%), followed by psychotropic medications (49%), BI (32%), and CAM (16%) (Table 2).
| Characteristic | N | % |
|---|---|---|
| Age (M±SD) | 8.9±1.5 | |
| Gender | ||
| Female | 135 | 18 |
| Male | 586 | 82 |
| Race-ethnicity | ||
| Non-Hispanic white | 519 | 73 |
| Hispanic | 73 | 10 |
| Non-Hispanic black | 47 | 7 |
| Non-Hispanic other | 75 | 11 |
| Health insurance | ||
| Any private | 487 | 69 |
| Public only or uninsured | 215 | 31 |
| Functional limitations | ||
| No | 269 | 37 |
| Yes | 453 | 63 |
| Household income (percentage of FPL)a | ||
| 0–99 | 106 | 15 |
| 100–199 | 149 | 21 |
| 200–399 | 245 | 34 |
| ≥400 | 222 | 31 |
| Parent education | ||
| High school or less | 108 | 15 |
| More than high school | 614 | 85 |
| U.S. Census region | ||
| Northeast | 138 | 19 |
| Midwest | 171 | 24 |
| South | 198 | 27 |
| West | 215 | 30 |
| Family structure | ||
| 2 parent, biological or adopted | 507 | 71 |
| Single mother | 106 | 15 |
| Other | 104 | 15 |
TABLE 1. Characteristics of 722 U.S. children ages six to 11 with autism spectrum disorder
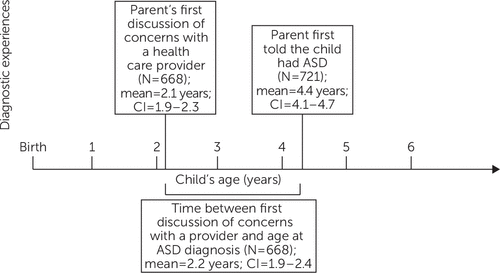
FIGURE 1. Delay in diagnosis of autism spectrum disorder (ASD) among 722 U.S. children ages six to 11a
aData were missing for some children.
| Characteristic | Psychotropic medicationa | BIb | CAMc | School-based therapyd | ||||
|---|---|---|---|---|---|---|---|---|
| Weighted % | 95% CI | Weighted % | 95% CI | Weighted % | 95% CI | Weighted % | 95% CI | |
| Total | 49 | 41.9–55.1 | 32 | 26.4–38.3 | 16 | 12.4–21.3 | 79 | 72.7–83.9 |
| Age at ASD diagnosis | ||||||||
| <4 | 31 | 22.4–40.5 | 44 | 34.2–53.5 | 20 | 13.4–29.8 | 90 | 82.6–94.1 |
| ≥4 | 60 | 51.3–67.2 | 25 | 18.6–33.0 | 14 | 9.6–19.7 | 72 | 63.3–79.2 |
| Delay in ASD diagnosis | ||||||||
| <2 years | 41 | 32.2–50.3 | 39 | 30.7–48.4 | 11 | 7.7–15.6 | 83 | 74.4–89.1 |
| ≥2 years | 55 | 45.6–64.3 | 26 | 19.2–34.1 | 21 | 14.3–28.9 | 75 | 66.3–82.5 |
| Age | ||||||||
| 6–8 | 38 | 28.4–48.2 | 35 | 26.3–45.3 | 18 | 11.1–26.9 | 81 | 70.5–88.6 |
| 9–11 | 56 | 47.4–64.0 | 30 | 23.1–38.2 | 16 | 11.0–21.6 | 77 | 69.5–83.7 |
| Gender | ||||||||
| Male | 50 | 42.5–56.8 | 32 | 25.5–38.7 | 16 | 11.5–20.8 | 80 | 73.6–85.9 |
| Female | 44 | 28.0–60.8 | 34 | 21.4–48.2 | 20 | 10.2–35.0 | 71 | 56.0–81.7 |
| Race-ethnicity | ||||||||
| Non-Hispanic white | 50 | 42.2–57.3 | 41 | 33.3–48.2 | 18 | 13.1–23.1 | 76 | 68.6–82.4 |
| Hispanic | 47 | 28.6–65.4 | 17 | 7.8–34.4 | 18 | 7.2–38.3 | 78 | 56.2–90.6 |
| Non-Hispanic black | 49 | 25.8–71.9 | 23 | 10.0–44.8 | 12 | 3.4–33.8 | 89 | 70.7–96.6 |
| Non-Hispanic other race | 47 | 27.5–67.1 | 18 | 9.5–31.4 | 12 | 5.6–23.6 | 86 | 66.1–94.8 |
| Health insurance | ||||||||
| Any private insurance | 47 | 39.1–54.6 | 31 | 24.5–38.1 | 16 | 11.5–22.7 | 78 | 70.1–83.9 |
| Public only or uninsured | 50 | 37.3–61.9 | 35 | 24.6–48.0 | 17 | 10.4–26.0 | 80 | 68.3–88.2 |
| Functional limitations | ||||||||
| No | 44 | 33.7–55.1 | 26 | 17.4–35.8 | 13 | 7.9–21.1 | 66 | 54.6–76.0 |
| Yes | 51 | 42.8–59.5 | 36 | 28.9–44.3 | 18 | 13.1–25.1 | 87 | 81.6–91.3 |
| Household income (percentage of FPL)e | ||||||||
| 0–99 | 68 | 50.9–80.6 | 28 | 14.8–47.5 | 20 | 9.8–35.4 | 72 | 52.6–85.4 |
| 100–199 | 49 | 34.8–63.8 | 33 | 21.2–46.9 | 10 | 5.5–18.3 | 91 | 83.2–95.2 |
| 200–399 | 42 | 32.0–52.3 | 37 | 27.8–47.3 | 15 | 8.9–22.8 | 85 | 75.5–91.0 |
| ≥400 | 43 | 31.8–54.8 | 28 | 19.4–38.8 | 21 | 13.2–32.9 | 66 | 52.9–77.1 |
| Parent education | ||||||||
| High school or less | 56 | 40.0–70.4 | 28 | 16.0–44.1 | 6 | 3.0–12.5 | 92 | 81.1–97.2 |
| More than high school | 46 | 39.2–53.2 | 33 | 27.2–40.0 | 20 | 14.7–25.7 | 75 | 67.5–80.7 |
| U.S. Census region | ||||||||
| Northeast | 37 | 24.2–51.0 | 46 | 33.1–59.5 | 12 | 6.8–21.2 | 87 | 76.1–93.0 |
| Midwest | 56 | 43.2–67.6 | 38 | 26.1–51.1 | 17 | 9.9–28.0 | 76 | 61.4–86.1 |
| South | 54 | 42.1–65.4 | 23 | 15.6–33.6 | 11 | 6.5–17.4 | 79 | 70.1–85.9 |
| West | 40 | 28.0–54.1 | 27 | 17.5–39.3 | 29 | 17.4–44.3 | 75 | 57.3–87.3 |
| Family structure | ||||||||
| 2 parent, biological or adopted | 43 | 35.0–50.4 | 34 | 27.4–41.7 | 18 | 12.7–23.7 | 78 | 70.9–84.2 |
| Single mother | 56 | 39.8–71.7 | 35 | 21.1–53.0 | 20 | 9.8–36.3 | 78 | 59.4–89.3 |
| Other | 58 | 41.2–73.1 | 22 | 12.1–36.0 | 10 | 4.9–18.2 | 84 | 67.6–92.6 |
TABLE 2. Receipt of health services by U.S. children ages six to 11 with autism spectrum disorder (ASD), by characteristic
Bivariate and multivariable analyses showed significant associations between the dichotomous measure of age at ASD diagnosis and use of psychotropic medication, BI, and school-based therapy (Tables 2 and 3). The strongest association was with use of school-based therapy: children with older ASD diagnosis ages had lower odds of current school-based therapy use versus children with younger diagnosis ages (72% and 90%, respectively; adjusted odds ratio [AOR]=.38). BI use was also less likely among children diagnosed at older versus younger ages (25% and 44%, respectively; AOR=.55). Psychotropic medication use was more likely among children diagnosed at older versus younger ages (60% and 31%, respectively; AOR=3.09).
| Measure | Psychotropic medicationb | BIc | CAMd | School-based therapye | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | AORf | 95% CI | p | OR | 95% CI | AORf | 95% CI | p | OR | 95% CI | AORf | 95% CI | p | OR | 95% CI | AORf | 95% CI | p | |
| Age at ASD diagnosis ≥4 (reference: <4) | 3.31 | 1.92–5.70 | 3.09 | 1.77–5.39 | <.001 | .44 | .25–.76 | .55 | .31–.97 | .039 | .63 | .32–1.21 | .84 | .42–1.69 | .628 | .29 | .14–.61 | .38 | .18–.83 | .014 |
| Delay in ASD diagnosis ≥2 years (reference: <2 years) | 1.77 | 1.03–3.03 | 1.69 | .97–2.94 | .063 | .54 | .32–.93 | .66 | .38–1.13 | .131 | 2.09 | 1.15–3.80 | 2.81 | 1.50–5.73 | .001 | .62 | .32–1.23 | .59 | .30–1.15 | .12 |
TABLE 3. Odds of health services utilization among U.S. children ages six to 11 with autism spectrum disorder (ASD), by age at ASD diagnosis and delay in ASD diagnosisa
When age at ASD diagnosis was treated as a continuous variable in multivariable models, there was a positive association between increasing age at diagnosis and psychotropic medication use (p=.001) (Table 4). There was a marginal negative association between age at ASD diagnosis and BI use (p=.07) and no significant associations between age at ASD diagnosis and use of CAM or school-based therapy.
| Measure | Psychotropic medicationb | BIc | CAMd | School-based therapye | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p | Coefficient | 95% CI | p | Coefficient | 95% CI | p | Coefficient | 95% CI | p | |
| Age at ASD diagnosis | .38 | .15 to .61 | .001 | −.22 | −.45 to .02 | .07 | −.18 | −.50 to .13 | .27 | −.22 | −.53 to .09 | .17 |
| Delay in ASD diagnosis | −.03 | −.22 to .15 | .72 | .04 | −.14 to .23 | .64 | .32 | .08 to .56 | .009 | .05 | −.15 to .26 | .61 |
TABLE 4. Association of age at autism spectrum disorder (ASD) diagnosis and delay in ASD diagnosis with utilization of health services among U.S. children ages six to 11 years with ASDa
Delay in ASD diagnosis had a different effect on use of services compared with age at ASD diagnosis. CAM use, which had no significant association with diagnosis age, was nearly twice as common among children with longer versus shorter delays (21% and 11%, respectively; AOR=2.81) (Tables 2 and 3). When diagnostic delay was treated as a continuous variable, there was a significant association with CAM use: as diagnostic delay increased, the likelihood of current CAM use increased (p=.009) (Table 4). Psychotropic medication use had a positive bivariate association with diagnostic delay, but the relationship was of borderline significance (p=.063) in multivariable logistic regression results (Table 3) and was nonsignificant when delay was treated as continuous (Table 4).
We considered whether the relationship between diagnostic delay and services receipt was affected by functional limitations status. As diagnostic delay increased, children with functional limitations became significantly less likely than children without functional limitations to receive school-based therapy (p=.04). [A figure depicting the relationship between probability of receiving school-based therapy and diagnostic delay among children with functional limitations is available as an online supplement to this article.] Functional limitations status did not modify the relationship between diagnosis delay and receipt of psychotropic medications, CAM, or BI.
The interaction between age at ASD diagnosis and delay in ASD diagnosis did not modify services use; that is, the relationship between diagnosis age and services use was not significantly different among children with shorter versus longer diagnostic delays. There was a significant negative interaction between diagnostic delay and ASD diagnosis length in relation to BI use: as both length of ASD diagnosis and diagnostic delay increased, likelihood of current BI use among children decreased. There were no significant interactions between diagnosis age and functional limitations status, comorbid intellectual disability and diagnosis age, or comorbid intellectual disability and diagnostic delay in terms of subsequent services use.
Discussion
This study’s goal was to investigate whether timeliness of ASD diagnosis was associated with subsequent ASD-related health services use. Because both early ASD diagnosis and prompt ASD diagnosis are important public health goals (35), we considered timeliness of ASD diagnosis in two ways: diagnosis at a younger versus older age and a longer versus shorter diagnostic delay. Results suggested that children diagnosed at older ages were less likely to currently use ASD-related therapy services and were more likely to take psychotropic medication compared with children diagnosed at younger ages. Results also suggested that children experiencing longer diagnostic delays were more likely to use CAM compared with children experiencing shorter delays. Because analyses controlled for current age and functional limitations status, differences are unlikely to be related to age cohort effects or lower ASD severity among children with late or delayed diagnoses. In fact, results revealed that children with ASD and functional limitations may be especially likely to receive no school-based therapy services when diagnosis is delayed.
Although the optimal type and amount of ASD therapy remain unclear, there is growing consensus that early therapy benefits children and families (40). It is therefore concerning that nearly a quarter of the elementary school–aged children studied were receiving no school-based therapy, and over half were not receiving BI. Among children who were four years old or older at diagnosis, who constituted approximately 50% of the sample, current therapy use was even lower. Instead, children diagnosed at older ages were more likely to receive psychotropic medications, which generally do not treat core ASD features. Similarly, children with long diagnostic delays were more likely than children with shorter delays to use CAM. Children with long delays and functional limitations (who may have the greatest therapy needs) had some of the lowest rates of use of school-based therapies. Together, these results suggest that families who receive later ASD diagnoses are less likely to use evidence-based therapy directed at core ASD symptoms and more likely to use alternative treatments.
These findings should interest providers and policy makers. Previous research has indicated that most parents delay only a few months before talking to providers about developmental concerns; however, substantial delays occur after initial provider conversations (10). This study adds to the literature by suggesting that ASD diagnostic delays are also associated with long-term treatment differences. Results suggest that if long-term ASD therapy use is a priority, payers and policymakers may need to proactively accelerate diagnosis by incentivizing screening or enhancing case management of children at high risk of diagnostic delays. From a population standpoint, as children receive earlier ASD diagnoses, payers may expect changes in service use patterns toward more therapy use and less pharmacology.
Although ASD diagnostic delays may play a causal role in subsequent services use, alternative explanations of study findings are plausible. For instance, families who receive a delayed diagnosis may be less connected to the health care system for a number of reasons, such as skepticism about effectiveness of conventional care and financial or geographic barriers to accessing care, making them less likely to seek conventional services and more likely to pursue CAM. Even if one were to assume that later diagnosis causes subsequent decreased conventional service use, the mechanism whereby that occurs is unclear. Pathways did not collect information about what therapy services parents were offered, why parents chose or rejected any particular services, or whether current services were specifically for ASD versus some other developmental or behavioral problem. Parents’ beliefs about ASD may play a role in subsequent treatment decisions (34,41); research exploring parents’ beliefs regarding treatment or longitudinal studies following families’ treatment decisions may elucidate these findings. Future research should explore why children were using certain therapies at high rates—for example, psychotropic medications, which generally are not indicated for ASD.
The study had other limitations. Pathways is based on parent report; consequently, ASD diagnoses were not verified. Few data exist regarding validity of parent-reported ASD diagnoses; however, national parent-reported surveys have produced prevalence estimates similar to studies that used more rigorous methods of ASD verification (2). There was no way to validate type or frequency of service use, although studies have revealed parents to be reliable sources of information about medication and health service use among typically developing children (42–45). Recall bias may also limit findings: parents may have had difficulty recalling the timing of their first conversation with a provider about developmental concerns or of the ASD diagnosis. To limit recall bias and account for imprecise date reporting, this study reported age in years and assessed only elementary school–aged children. Nonetheless, correlations found in the data may be due to biases in parent reporting rather than true associations—for example, perhaps parents who reported earlier ASD diagnoses also more often reported certain details about their child’s current therapy compared with other parents. Also, because the study rounded age at ASD diagnosis and delay in ASD diagnosis to the highest whole year of age, findings may underestimate diagnosis age and diagnostic delay for some children.
This study focused on children with ASD, regardless of whether the children had a comorbid intellectual disability or developmental delay. Children with ASD and intellectual disability or developmental delay may have used different services than other children with ASD (20). Sensitivity analyses did not suggest that the presence of these comorbid conditions was salient to the studied outcomes. We used current functional status as a covariate in models to adjust for confounding by severity of ASD; however, current functional status may have differed from functional status at the time of the initial provider conversation, the ASD diagnosis, or both. Finally, the sample from Pathways and the NS-CSHCN could be subject to nonresponse bias from either survey.
Conclusions
To our knowledge, this is the first study indicating that children diagnosed as having ASD at older ages or after experiencing longer delays used different health services than other children with ASD. Results suggest that children with diagnostic delays or older diagnosis age were less likely to use conventional ASD therapy and were more likely to use alternate treatments, such as medication or CAM. Results also suggest that efforts to increase early ASD diagnosis may result in greater ASD-related therapy use and improved functional outcomes for children with ASD.
1 Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, Va, American Psychiatric Association, 2013Google Scholar
2 Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report Surveillance Summaries 63:1–21, 2014Medline, Google Scholar
3 : The onset of autism: patterns of symptom emergence in the first years of life. Autism Research 1:320–328, 2008Crossref, Medline, Google Scholar
4 : Autism spectrum disorder and autistic traits in the Avon Longitudinal Study of Parents and Children: precursors and early signs. Journal of the American Academy of Child and Adolescent Psychiatry 51:249–260, 2012Crossref, Medline, Google Scholar
5 : Behavioral treatments in autism spectrum disorder: what do we know? Annual Review of Clinical Psychology 6:447–468, 2010Crossref, Medline, Google Scholar
6 : Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 10:CD009260, 2012Medline, Google Scholar
7 : Meta-analysis of early intensive behavioral intervention for children with autism. Journal of Clinical Child and Adolescent Psychology 53:439–450, 2009Google Scholar
8 Therapies for Children With Autism Spectrum Disorders: Comparative Effectiveness Review. AHRQ pub no11-EHC029-1. Rockville, Md, Agency for Healthcare Research and Quality, 2014. http://effectivehealthcare.ahrq.gov/ehc/products/544/1945/autism-update-report-140806.pdfGoogle Scholar
9 : “You know what boys are like”: pre-diagnosis experiences of parents of children with autism spectrum conditions. British Journal of General Practice 62:e378–e383, 2012Google Scholar
10 : Parental concerns, provider response, and timeliness of autism spectrum disorder diagnosis. Journal of Pediatrics 166:1431–1439.e1, 2015Crossref, Medline, Google Scholar
11 : The diagnosis of autism and Asperger syndrome: findings from a survey of 770 families. Developmental Medicine and Child Neurology 41:834–839, 1999Crossref, Medline, Google Scholar
12 : Identification and evaluation of children with autism spectrum disorders. Pediatrics 120:1183–1215, 2007Crossref, Medline, Google Scholar
13 SDBP Response to USPSTF Draft Recommendation on Screening for ASD in Young Children. McLean, Va, Society for Developmental and Behavioral Pediatrics, 2015. https://www.sdbp.org/pdf/USPSTF_response.pdfGoogle Scholar
14 : Parent and pediatrician perspectives regarding the primary care of children with autism spectrum disorders. Journal of Autism and Developmental Disorders 43:964–972, 2013Crossref, Medline, Google Scholar
15 : Pediatrician identification of Latino children at risk for autism spectrum disorder. Pediatrics 132:445–453, 2013Crossref, Medline, Google Scholar
16 : Children’s compliance with American Academy of Pediatrics’ well-child care visit guidelines and the early detection of autism. Journal of Autism and Developmental Disorders 43:2844–2854, 2013Crossref, Medline, Google Scholar
17 : Barriers to evaluation for early intervention services: parent and early intervention employee perspectives. Academic Pediatrics 12:551–557, 2012Crossref, Medline, Google Scholar
18 : Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. Journal of Developmental and Behavioral Pediatrics 27(suppl):S79–S87, 2006Crossref, Medline, Google Scholar
19 : Management of children with autism spectrum disorders. Pediatrics 120:1162–1182, 2007Crossref, Medline, Google Scholar
20 : Service and treatment use among children diagnosed with autism spectrum disorders. Journal of Developmental and Behavioral Pediatrics 36:98–105, 2015Crossref, Medline, Google Scholar
21 : Complementary and alternative medicine treatments for children with autism spectrum disorders. Child and Adolescent Psychiatric Clinics of North America 24:117–143, 2015Crossref, Medline, Google Scholar
22 : Sensory integration therapies for children with developmental and behavioral disorders. Pediatrics 129:1186–1189, 2012Crossref, Medline, Google Scholar
23 : Disparities in diagnoses received prior to a diagnosis of autism spectrum disorder. Journal of Autism and Developmental Disorders 37:1795–1802, 2007Crossref, Medline, Google Scholar
24 : Are minority children disproportionately represented in early intervention and early childhood special education? Educational Researcher 41:339–351, 2012Crossref, Medline, Google Scholar
25 2009–10 National Survey of Children With Special Health Care Needs (questionnaire). Atlanta, National Center for Health Statistics, Dec 14, 2011. http://www.cdc.gov/nchs/data/slaits/NS_CSHCN_Questionnaire_09_10.pdfGoogle Scholar
26 Survey of Pathways to Diagnosis and Services. Atlanta, National Center for Health Statistics, 2012. http://www.cdc.gov/nchs/slaits/spds.htmGoogle Scholar
27 : Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambulatory Pediatrics 2:38–48, 2002Crossref, Medline, Google Scholar
28 Frequently Asked Questions: 2009–2010 National Survey of CSHCN. Atlanta, National Center for Health Statistics, 2013. http://www.cdc.gov/nchs/data/slaits/NSCSHCNfaqs2009.pdfGoogle Scholar
29 Frequently Asked Questions: 2011 Survey of Pathways to Diagnosis and Services, Atlanta, National Center for Health Statistics, April 24, 2012. http://www.cdc.gov/nchs/data/slaits/PathwaysFAQ.pdfGoogle Scholar
30 : Parental recognition of developmental problems in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders 37:62–72, 2007Crossref, Medline, Google Scholar
31 : Diagnosis of autism spectrum disorders in 2-year-olds: a study of community practice. Journal of Child Psychology and Psychiatry, and Allied Disciplines 54:178–185, 2013Crossref, Medline, Google Scholar
32 : Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. Journal of Child Psychology and Psychiatry, and Allied Disciplines 54:582–590, 2013Crossref, Medline, Google Scholar
33 : A national profile of the health care experiences and family impact of autism spectrum disorder among children in the United States, 2005–2006. Pediatrics 122:e1149–e1158, 2008Crossref, Medline, Google Scholar
34 : Parent health beliefs, social determinants of health, and child health services utilization among US school-age children with autism. Journal of Developmental and Behavioral Pediatrics 36:146–157, 2015Crossref, Medline, Google Scholar
35 Interagency Autism Coordinating Committee Strategic Plan for Autism Spectrum Disorder Research—2013 Update. Washington, DC, US Department of Health and Human Services, 2013. https://iacc.hhs.gov/publications/strategic-plan/2013/Google Scholar
36 : Taking stock of the CSHCN screener: a review of common questions and current reflections. Academic Pediatrics 15:165–176, 2015Crossref, Medline, Google Scholar
37 : Access to diagnosis and treatment services among Latino children with autism spectrum disorders. Intellectual and Developmental Disabilities 51:141–153, 2013Crossref, Medline, Google Scholar
38 : Health care of Latino children with autism and other developmental disabilities: quality of provider interaction mediates utilization. American Journal on Intellectual and Developmental Disabilities 117:304–315, 2012Crossref, Medline, Google Scholar
39 : Barriers to detection, help-seeking, and service use for children with ADHD symptoms. Journal of Behavioral Health Services and Research 30:176–189, 2003Crossref, Medline, Google Scholar
40 : Management of autism spectrum disorders in primary care. Pediatric Annals 38:42–49, 2009Crossref, Medline, Google Scholar
41 : Treatment choices in autism spectrum disorder: the role of parental illness perceptions. Research in Developmental Disabilities 31:817–828, 2010Crossref, Medline, Google Scholar
42 : Parents as partners in obtaining the medication history. Journal of the American Medical Informatics Association 12:299–305, 2005Crossref, Medline, Google Scholar
43 : Validity of maternal report of acute health care use for children younger than 3 years. Archives of Pediatrics and Adolescent Medicine 159:167–172, 2005Medline, Google Scholar
44 : Parents as direct contributors to the medical record: validation of their electronic input. Annals of Emergency Medicine 35:346–352, 2000Crossref, Medline, Google Scholar
45 : Parents were accurate proxy reporters of urgent pediatric asthma health services: a retrospective agreement analysis. Journal of Clinical Epidemiology 60:1176–1183, 2007Crossref, Medline, Google Scholar



