The RAISE Connection Program: Psychopharmacological Treatment of People With a First Episode of Schizophrenia
Abstract
Objective:
This study examined the adherence of psychiatrists to the Schedule of Recommended First and Second Line Antipsychotic Medications (“Antipsychotic Schedule”), which was implemented in two Recovery After an Initial Schizophrenia Episode (RAISE) Connection Program Implementation and Evaluation Study clinics.
Methods:
Sixty-five individuals with a first episode of psychosis were enrolled in the RAISE Connection Program clinics. Two psychiatrists received training and ongoing consultation on use of a shared decision-making approach to prescribing antipsychotic medications according to the Antipsychotic Schedule. Information about participants, prescribed antipsychotic medications, and completion of side-effect assessments were obtained from standardized research assessments and chart extractions. Descriptive statistics were used to characterize the extent to which patterns of antipsychotic prescribing and side-effect monitoring were consistent with the Antipsychotic Schedule.
Results:
Ninety-two percent of participants were prescribed an antipsychotic medication and received the medication on 76%±35% of the days they were in treatment. Seventy-seven percent of participants were prescribed at least one Antipsychotic Schedule first-line antipsychotic, 20% were prescribed olanzapine, and 10% received a trial of clozapine. Regarding monitoring for metabolic side effects, 92% of participants had at least one weight recorded, 72% had at least one blood glucose measure recorded, and 62% had at least one lipid profile recorded.
Conclusions:
In the context of a study in which training and ongoing clinical supervision by experts was provided to psychiatrists and shared decision making was encouraged, antipsychotic prescribing patterns closely adhered to recommendations established by the RAISE Connection Program.
Multiple studies have examined the use of antipsychotic medications by people experiencing a first episode of schizophrenia or a related disorder (1–8). These studies suggest that up to 80% of these individuals will respond to antipsychotic treatment, they are more likely to respond to lower doses, there are minimal efficacy differences among the various antipsychotics but substantial differences in their side-effect profiles, and adolescents are more sensitive than adults to adverse effects.
However, many people experiencing a first episode of schizophrenia will opt not to adhere to their prescribed antipsychotic regimen (9–14). Adverse effects are a major obstacle to ongoing treatment with these agents (9), with metabolic and neurological side effects, prolactin elevation, and sedation the most common and distressing. Nonadherence may also be related to not believing one is ill, substance abuse, and actual or perceived lack of medication efficacy (9–16). Antipsychotic treatment nonadherence can lead to inadequate symptom control, which can compromise the ability of the person to fully engage in treatment.
Emerging evidence suggests that the use of a shared decision-making approach may increase the likelihood of treatment participation. Shared decision making is a collaborative process in which both consumers and providers are viewed as experts and equal partners in sharing knowledge and information with each other and actively participating in treatment decisions (17,18). The role of the treatment provider is to educate the consumer and family concerning available evidence-based treatments, to share his or her clinical experience in using these treatments, to acknowledge and help clarify consumer preferences and values in regard to treatment, and to empower consumers to take an active role in the treatment decision-making process. Consumers are the experts on their own values, lived experience with the disorder and treatments, and treatment preferences and goals (19). A shared decision-making approach reflects current efforts to increase consumers’ involvement in their own care, which is in line with a recovery-oriented, client-centered approach designed to foster treatment engagement (20–22).
We developed the Recovery After an Initial Schizophrenia Episode Connection Program (RAISE CP) Schedule of Recommended First and Second Line Antipsychotic Medications (“Antipsychotic Schedule”) to guide the selection of antipsychotic treatment within a shared decision-making framework. We conducted a longitudinal, observational study to evaluate the adherence of psychiatrists to the Antipsychotic Schedule for individuals with first-episode schizophrenia participating in the RAISE CP Implementation and Evaluation Study (23).
Methods
An overview of the RAISE CP Implementation and Evaluation Study and primary and secondary study outcomes are available elsewhere (23–26). In brief, RAISE CP was a multidisciplinary and multielement program designed to reduce morbidity and disability among individuals experiencing a first episode of psychosis suggestive of schizophrenia. Treatment was offered for up to two years and was based on a critical time intervention framework (27) that included community-based outreach. RAISE CP treatment teams comprised a team leader, an individual placement and support worker, a recovery coach, and a psychiatrist, who was responsible for psychiatric assessment and medication management. The study was conducted at two sites, Baltimore and Manhattan, between July 2011 and March 2013. All participants (and, for minors, the participant’s parent or guardian) provided written informed consent; minors provided assent. The New York State Psychiatric Institute and University of Maryland Institutional Review Boards approved study procedures.
The Antipsychotic Schedule was used to guide antipsychotic medication management (Table 1). The included medications had been shown to be effective for first-episode schizophrenia and, when prescribed appropriately, had relatively tolerable side-effect profiles, thereby increasing the likelihood that the treatment experience would facilitate treatment engagement (1). Both oral and long-acting injectable (LAI) antipsychotics were included as first-line treatment options to increase the probability that there would be an available agent to meet the particular needs and preferences of a participant (28). We included most LAI antipsychotics, because these agents are relatively underutilized in the United States, especially by individuals with first-episode psychosis. We did not include extended-release olanzapine because of the requirement that individuals who receive this agent must be observed for three hours after the injection. Oral olanzapine was recommended as a second-line treatment option because of its increased liability for weight gain and other metabolic adverse effects (8). The recommended dosage range for all first- and second-line treatment options was the lower half of the schizophrenia Patient Outcomes Research Team (PORT) recommended range for each agent for individuals with multiepisode schizophrenia (1). If distressing psychopathology persisted after two adequate antipsychotic trials, it was recommended that participants be offered a clozapine trial.
| Antipsychotic | Dosage range and frequency of administration |
|---|---|
| First-line treatment options | |
| Oral formulations | |
| Aripiprazole | 5–15 mg per day |
| Loxapine | 10–25 mg per day |
| Lurasidone | 40–80 mg per day |
| Perphenazine | 4–12 mg per day |
| Risperidone | 1–4 mg per day |
| Long-acting injectable formulations | |
| Fluphenazine decanoate | 6.25–12.5 mg every 2 weeks |
| Haloperidol decanoate | 25–100 mg every 4 weeks |
| Paliperidone palmitate | 39–117 mg every 4 weeks |
| Risperidone microspheres | 12.5–50 mg every 2 weeks |
| Second-line treatment option | |
| Olanzapine | 5–15 mg per day |
TABLE 1. The RAISE Connection Program Schedule of Recommended First and Second Line Antipsychotic Medications
Other agents (for example, oral haloperidol) were not included in the Antipsychotic Schedule because of concerns about longer-term efficacy and neurological side effects relative to other medications included in the Antipsychotic Schedule (1) or because of potentially burdensome administration for younger individuals (for example, administering ziprasidone with food).
RAISE CP psychiatrists were expected to implement the Antipsychotic Schedule by using a shared decision-making approach with participants and family members. The overarching expectation was that antipsychotic medications would be prescribed continuously to all individuals willing to accept such treatment. Measurement-based care principles were also employed to guide antipsychotic treatment management. Psychiatrists were asked to regularly evaluate treatment response and the occurrence of specific side effects. Psychiatrists used abbreviated (four-item) objective assessments, adapted from validated scales of positive (29), negative (30), and depressive (31) symptoms, to assess efficacy; a checklist to evaluate participant-reported antipsychotic side effects; and abbreviated assessments of extrapyramidal side effects (three items) (32) and tardive dyskinesia (one item) (33) to assess neurological side effects. Symptom and side-effect assessments were scheduled for the first visit, monthly for the first three months, and every 90 days thereafter. To evaluate possible weight gain and the development of metabolic abnormalities, it was recommended that psychiatrists measure weight at baseline and then monthly and that blood glucose and lipid profile measures be obtained at baseline, two months later, and annually thereafter (25).
A two-day training session, followed by ongoing consultation with experts in the psychopharmacological management of psychosis, treatment of adolescents with psychotic disorders, and shared decision making, was provided to RAISE CP psychiatrists throughout the study. The consultation consisted of bimonthly hour-long conference calls and, as needed, telephone and e-mail consultations between the scheduled calls.
Data Collection and Analyses
Information on previous antipsychotic prescriptions was collected through research interviews (23). Information on prescribed antipsychotic medications, including start and stop dates, dosages, and dates on which side-effect assessments were completed, were obtained from chart extractions (25).
We used descriptive statistics to describe the sample and to characterize antipsychotic prescriptions for participants immediately before entry and while they received treatment in the RAISE CP, including whether first- or second-line Antipsychotic Schedule antipsychotics were prescribed. We also computed the dosage mean, standard deviation, and median for each antipsychotic prescribed and determined whether participants were prescribed dosages in the lower half of the ranges recommended by the schizophrenia PORT (1). These dose analyses were conducted only for medications for which there was more than one 30-day prescription.
To examine overall antipsychotic “coverage,” we computed the percentage of days that each participant was prescribed at least one antipsychotic medication, excluding days the participant was hospitalized. We excluded the first month of treatment from these calculations, because most participants entered the study posthospitalization with an active antipsychotic prescription and did not need a prescription from the team psychiatrist. We also excluded months 21–24 because of a small sample size.
A number of participants were prescribed more than one antipsychotic during their study participation. Therefore, we calculated the median number of days until a participant was prescribed a second and third different antipsychotic medication, regardless of whether the antipsychotic was included in the Antipsychotic Schedule. These analyses included only participants who were prescribed an antipsychotic by the team psychiatrist. We also calculated the frequency of antipsychotic polypharmacy, which was examined by using two different thresholds—that is, the concurrent prescription of two or more different antipsychotic medications for at least 30 and at least 60 days. In these analyses, different formulations of the same medication were considered the same drug.
Finally, we evaluated adherence to the recommended frequency of side-effect assessments by examining whether at least one side-effect checklist, weight measurement, fasting blood glucose or glycosylated hemoglobin level, or lipid profile was recorded in the medical record at any time during the study.
Results
Sixty-five participants were enrolled in the study; their demographic and clinical characteristics can be found in the primary report (23). In brief, their mean age was 22.2±4.2 years and 63% (N= 41) were male. A total of 28 (43%) identified themselves as black, 25 (39%) as white, four (6%) as Asian or Pacific Islander, one (2%) as American Indian or Alaska Native, and one (2%) as multiracial; six (9%) did not specify a group. Sixteen participants (25%) described themselves as Latino or of Hispanic origin. The Positive and Negative Syndrome Scale (34) mean total score for participants was 64.0±14.3 (possible scores range from 30 to 210, with higher scores indicating greater symptom severity). Over the course of their treatment, they experienced significant reductions in positive and negative symptoms and improvements in social and occupational functioning and reported high rates of shared decision making, with over 90% of participants reporting that their psychiatrist involved them in decisions about their medications (23,25).
Fifty-six (86%) of the 65 participants entered the study already prescribed an antipsychotic medication. Thirty-seven (66%) were prescribed an Antipsychotic Schedule first-line antipsychotic, 17 (30%) were prescribed a non–first-line antipsychotic medication, two (4%) were prescribed both first-line and non–first-line antipsychotic medications, and nine (14%) were not prescribed any psychotropic medications at study entry. Among these nine individuals, two were never prescribed an antipsychotic during the study, two were prescribed a first-line antipsychotic, two received the second-line antipsychotic olanzapine, and three were prescribed a non–first-line and non–second-line antipsychotic.
During the study period, 60 (92%) participants were prescribed an antipsychotic and five (8%) were never prescribed an antipsychotic medication. The mean percentage of days all participants were prescribed at least one antipsychotic medication was 76%± 35%. Figure 1 suggests that the percentage of participants who received an antipsychotic remained consistent over the course of the study. Among the 60 individuals who were prescribed an antipsychotic during the study period, the most commonly prescribed antipsychotics were aripiprazole (N=26), oral risperidone (N=24), quetiapine (N=14), oral haloperidol (N=13), olanzapine (N=12), perphenazine (N=11), haloperidol LAI (N=8), clozapine (N=6), and others (N≤4).
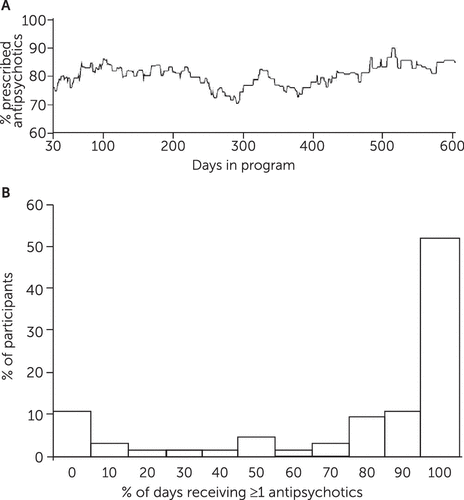
FIGURE 1. Percentage of RAISE Connection Program participants prescribed antipsychotics (panel A) and the percentage of study days that participants received at least one antipsychotic (panel B)
The recommended dosage range, the mean and median prescribed doses, and the prescribed dosage range for each antipsychotic with more than one 30-day prescription are displayed in Table 2. In general, mean prescribed dosages were within the recommended ranges, except for perphenazine (16.9 mg), which was prescribed above the recommended range, and for two antipsychotics not included in the Antipsychotic Schedule, oral haloperidol (12.0 mg) and quetiapine (269.9 mg), in which prescribed doses were above and below the recommended ranges, respectively.
| Antipsychotic | Recommended dosage range (mg) | N of drug daysa | Dose (mg) | Median dose (mg) | Dosage range (mg) | |
|---|---|---|---|---|---|---|
| M | SD | |||||
| Aripiprazole | 5–15 | 7,167 | 12.95 | 8.73 | 10 | 2–40 |
| Clozapine | 200–600 | 1,901 | 407.21 | 121.06 | 400 | 12.5–700 |
| Haloperidol (oral) | 2–6 | 3,145 | 12.02 | 5.55 | 10 | 2–20 |
| Haloperidol decanoate | 25–100 | 1,957 | 93.71 | 17.26 | 100 | 50–125 |
| Loxapine | 10–25 | 57 | 18.60 | 13.69 | 10 | 10–50 |
| Lurasidone | 40–80 | 414 | 44.15 | 12.22 | 40 | 40–80 |
| Olanzapine | 5–15 | 2,707 | 9.40 | 7.17 | 5 | 2.5–30 |
| Perphenazine | 4–12 | 2,392 | 16.88 | 8.61 | 16 | 2–40 |
| Quetiapine | 300–600 | 3,421 | 269.88 | 176.25 | 200 | 25–800 |
| Risperidone (oral) | 1–4 | 4,056 | 3.09 | 1.34 | 3 | .5–6 |
| Risperidone microspheres | 12.5–50 | 1,291 | 31.56 | 12.95 | 25 | 12.5–50 |
TABLE 2. Prescribed dosages of antipsychotic medications in the RAISE Connection Program
Among the 60 individuals prescribed antipsychotics, 77% (N=46) were prescribed at least one Antipsychotic Schedule first-line antipsychotic. This included seven of 16 individuals who entered RAISE CP with a prescribed non–first-line antipsychotic and three of seven who entered the program not receiving any antipsychotic treatment. Twelve individuals (20%) were prescribed an LAI antipsychotic, all of which were Antipsychotic Schedule first-line treatments.
During their time in the study, almost half of the participants were treated with a second antipsychotic (N=29 of 60, 48%). The median time to the prescription of the second antipsychotic was 58 days. Most of these antipsychotics were first-line agents (N=23 of 29, 79%). Ten of 60 (17%) participants were treated with a third antipsychotic. The median time to the prescription of the third antipsychotic was 161 days.
During the study, 12 (20%) participants were prescribed olanzapine, including five individuals who entered RAISE CP while taking olanzapine and seven participants newly prescribed olanzapine during the study. In two of these seven cases, one or more first-line antipsychotics were prescribed prior to the olanzapine trial. If a participant did not respond to a second antipsychotic trial, RAISE CP psychiatrists were encouraged to consider prescribing clozapine. Six (10%) participants had at least one clozapine trial during the course of the study.
Less than a quarter of participants were prescribed nonrecommended antipsychotics during the study, with 13 of 60 (22%) prescribed oral haloperidol and 14 of 60 (23%) prescribed quetiapine. Of note, 69% (N=9) who were prescribed oral haloperidol and 57% (N=8) who were prescribed quetiapine were also prescribed one of the first-line antipsychotics at some other time during the study. At some point during the study, 14 (22%) participants were prescribed two concurrent antipsychotics for more than 30 consecutive days; the most commonly prescribed antipsychotic combination was oral or LAI haloperidol with quetiapine. Olanzapine was prescribed to two of the 14 individuals, but no participant received clozapine before the initiation of antipsychotic polypharmacy. The use of a 60-day criterion for defining polypharmacy produced similar results.
All 60 participants prescribed an antipsychotic had one or more medication side-effect checklists completed. Although 55 (92%) participants had at least one weight recorded, only 43 (72%) had at least one blood glucose measure and only 37 (62%) had at least one lipid profile recorded during their RAISE CP participation.
Discussion
In line with RAISE CP recommendations, most participants (92%) were prescribed an antipsychotic medication. The 76% average rate of antipsychotic coverage achieved likely reflects the use of a shared decision-making approach, in which participants were able to continue to receive RAISE CP services and meet with the psychiatrist, even if they did not receive antipsychotic treatment. These ongoing psychiatrist visits provided the opportunity for close monitoring of symptoms and a restart of antipsychotic medication if symptoms worsened, and they also facilitated the use of nonpharmacological approaches to symptom management, all of which were jointly agreed upon by the treatment team and the participant.
Over half of study participants entered the study already prescribed an Antipsychotic Schedule first-line antipsychotic, and almost 80% of those prescribed antipsychotics during the study received one of these agents, including 12 participants who were treated with an LAI antipsychotic. Although approximately 20% of participants received a nonrecommended antipsychotic, it is possible that some patients requested to continue on their previously prescribed medication. However, it should be noted that 60% to 75% of those who received nonrecommended antipsychotics were prescribed a first-line antipsychotic agent at some point during the study. Furthermore, only three antipsychotics were prescribed at mean dosages that fell outside of RAISE CP recommended ranges, and two of these agents were non–first-line medications. The large number of participants who were treated with a first-line antipsychotic within the prescribed dosage range supports the ability of psychiatrists to adhere to the Antipsychotic Schedule. Because the medications included in the Antipsychotic Schedule were selected for tolerability as well as efficacy (2–8), the extensive use of these first-line agents, within a shared decision-making framework, may have contributed to the high rate of engagement and retention of study participants, who remained in RAISE CP treatment for 91%±21% of the total possible time they could have received services (24).
Although the literature is clear that individuals experiencing a first episode of psychosis are likely to respond to antipsychotic treatment, almost half of the study participants received a second antipsychotic. A smaller percentage (17%) was treated with a third agent. In this study, only 20% of participants were prescribed the second-line antipsychotic olanzapine, a rate that was lower than that reported in the RAISE Early Treatment Program (ETP) study (32%) (35). Also consistent with the RAISE CP Antipsychotic Schedule, six participants received at least one clozapine trial. These data suggest that prescribers should be prepared to engage in ongoing shared decision making with patients in order to identify alternative medication choices consistent with their preferences.
Although the use of antipsychotic polypharmacy is not recommended, it was relatively common, with over 20% of participants treated concurrently with two antipsychotics at some point during their study participation. The 30- and 60-day criteria produced similar polypharmacy rates, which suggests that the observed cases were not examples of antipsychotic cross-titration. The antipsychotic polypharmacy rates were similar to those observed in the RAISE ETP Study (23%) (35). These data suggest that even early in the course of treatment, a subgroup of people with first-episode psychosis may experience inadequate symptom response, prompting the use of treatment regimens typically reserved for individuals who are later in the course of their illness, including treatment regimens for which evidence for efficacy is lacking and concerns about safety have been raised (that is, antipsychotic polypharmacy) (1).
Because antipsychotic medication side effects are major contributors to treatment nonadherence and can adversely affect health and well-being, the RAISE CP emphasized the role of the psychiatrist in monitoring the occurrence of side effects. Our ability to at least partially implement such monitoring was evidenced by the fact that that all participants who were prescribed an antipsychotic were systematically asked about side effects at least once during their time in the program. Although most participants had their weights recorded at least once, these measurements occurred less frequently than the benchmark of at least once per month for individuals prescribed an antipsychotic. Consistent with previous research (36), glucose and lipid level evaluations also fell short of the recommended frequency. This demonstrates that even specialized services may have difficulty attaining recommended antipsychotic side effect–monitoring goals. In this study, psychiatrists cited both logistical and patient barriers to adhering to the recommended monitoring schedule, including not having on-site phlebotomy or laboratory services. The addition of a nurse who can draw blood on site and develop a trusting relationship with individuals may address these barriers and has been implemented in current RAISE-based treatment teams.
This study had several limitations, including a relatively small sample, the observational design, and prescribing patterns reflecting practices at only two sites. Because the study lacked a comparison group, we cannot know if the extent of adherence to the Antipsychotic Schedule was a result of the training and ongoing consultation received by the psychiatrists or some other aspect of the structured environment of a research study. Also, although we were able to evaluate whether common antipsychotic side effects were assessed, we were unable to examine whether the recommended symptom assessments were completed; however, participants reported high rates of having their psychiatrist ask them about symptoms and side effects (25). Finally, we recognize that there may be disagreements regarding the inclusion and exclusion of specific agents in the Antipsychotic Schedule. For example, despite recommending LAI haloperidol as a first-line treatment, we elected not to include low-dose oral haloperidol because of controlled studies suggesting that long-term efficacy was not comparable to that of other agents included in the Antipsychotic Schedule and that haloperidol may be associated with more neurological side effects (1). However, a reasonable approach would have been to include oral haloperidol as a second-line treatment.
Conclusions
RAISE CP psychiatrists largely adhered to a schedule that recommended specific antipsychotic agents and dosage ranges to individuals experiencing a first episode of psychosis. The ability to adhere to the schedule was facilitated through the use of a shared decision-making framework to maintain participant engagement in treatment. The implementation of shared decision making was enhanced by training and clinical supervision sessions for psychiatrists led by experts in these approaches, including an individual with lived experience with the disorder. The extent to which these components of the approach to the pharmacological treatment of this population can be feasibly disseminated and implemented in regular clinical practice requires additional investigation.
1 : The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophrenia Bulletin 36:71–93, 2010Crossref, Medline, Google Scholar
2 : Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology 28:995–1003, 2003Crossref, Medline, Google Scholar
3 : Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. American Journal of Psychiatry 162:947–953, 2005Link, Google Scholar
4 : Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. American Journal of Psychiatry 163:2096–2102, 2006Link, Google Scholar
5 : Maintenance treatment with risperidone or low-dose haloperidol in first-episode schizophrenia: 1-year results of a randomized controlled trial within the German Research Network on Schizophrenia. Journal of Clinical Psychiatry 68:1763–1774, 2007Crossref, Medline, Google Scholar
6 : Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. American Journal of Psychiatry 164:1050–1060, 2007Link, Google Scholar
7 : Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 371:1085–1097, 2008Crossref, Medline, Google Scholar
8 : Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study. American Journal of Psychiatry 165:1420–1431, 2008Link, Google Scholar
9 : The Expert Consensus Guideline Series: adherence problems in patients with serious and persistent mental illness. Journal of Clinical Psychiatry 70(suppl 4):1–46, 2009Crossref, Medline, Google Scholar
10 : Predictors of medication discontinuation by patients with first-episode schizophrenia and schizoaffective disorder. Schizophrenia Research 57:209–219, 2002Crossref, Medline, Google Scholar
11 : A prospective evaluation of adherence to medication in first episode schizophrenia. European Psychiatry 21:29–33, 2006Crossref, Medline, Google Scholar
12 : Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. Journal of Clinical Psychiatry 69:106–113, 2008Crossref, Medline, Google Scholar
13 : Medication adherence of individuals with a first episode of psychosis. Acta Psychiatrica Scandinavica 106:286–290, 2002Crossref, Medline, Google Scholar
14 : Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. American Journal of Psychiatry 168:286–292, 2011Link, Google Scholar
15 : Clinical correlates of early medication adherence: West London first episode schizophrenia study. Acta Psychiatrica Scandinavica 108:439–446, 2003Crossref, Medline, Google Scholar
16 : Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophrenia Research 83:53–63, 2006Crossref, Medline, Google Scholar
17 : Shared decision-making and evidence-based practice. Community Mental Health Journal 42:87–105, 2006Crossref, Medline, Google Scholar
18 : Shared decision making and medication management in the recovery process. Psychiatric Services 57:1636–1639, 2006Link, Google Scholar
19 : Shared decision making in antipsychotic management. Journal of Psychiatric Practice 14:333–344, 2008Crossref, Medline, Google Scholar
20 Achieving the Promise: Transforming Mental Health Care in America. Pub no SMA-03-3832. Rockville, Md, Department of Health and Human Services, President’s New Freedom Commission on Mental Health, 2003Google Scholar
21 : The promise of shared decision making in mental health. Psychiatric Rehabilitation Journal 34:7–13, 2010Crossref, Medline, Google Scholar
22 : Disengagement from mental health treatment among individuals with schizophrenia and strategies for facilitating connections to care: a review of the literature. Schizophrenia Bulletin 35:696–703, 2009Crossref, Medline, Google Scholar
23 : Implementing coordinated specialty care for early psychosis: the RAISE Connection Program. Psychiatric Services 66:691–698, 2015Link, Google Scholar
24 : The RAISE Connection Program for early psychosis: secondary outcomes and mediators and moderators of improvement. Journal of Nervous and Mental Disease 203:365–371, 2015Crossref, Medline, Google Scholar
25 : Practical monitoring of treatment fidelity: examples from a team-based intervention for people with early psychosis. Psychiatric Services 66:674–676, 2015Link, Google Scholar
26 : State partnerships for first-episode psychosis services. Psychiatric Services 66:671–673, 2015Link, Google Scholar
27 : Critical Time Intervention: an empirically supported model for preventing homelessness in high risk groups. Journal of Primary Prevention 28:295–312, 2007Crossref, Medline, Google Scholar
28 : Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. a randomized clinical trial. JAMA Psychiatry 72:822–829, 2015Crossref, Medline, Google Scholar
29 : The Brief Psychiatric Rating Scale. Psychological Reports 10:799–812, 1962Crossref, Google Scholar
30 : Negative symptoms in schizophrenia: definition and reliability. Archives of General Psychiatry 39:784–788, 1982Crossref, Medline, Google Scholar
31 : A depression rating scale for schizophrenics. Schizophrenia Research 3:247–251, 1990Crossref, Medline, Google Scholar
32 : A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 212:11–19, 1970Crossref, Google Scholar
33 Guy W: ECDEU Assessment Manual for Psychopharmacology: Revised. DHEW pub no ADM 76-338. Rockville, Md, US Department of Health, Education and Welfare, Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976Google Scholar
34 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 13:261–276, 1987Crossref, Medline, Google Scholar
35 : Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. American Journal of Psychiatry 172:237–248, 2015Link, Google Scholar
36 : Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychological Medicine 42:125–147, 2012Crossref, Medline, Google Scholar



