Risk of Hospitalization and Use of First- Versus Second-Generation Antipsychotics Among Nursing Home Residents
Abstract
Objective
The study compared the risk of all-cause hospitalization associated with use of first- versus second-generation antipsychotics among elderly nursing home residents who were eligible for both Medicare and Medicaid.
Methods
A retrospective cohort study design was used to compare the risk of all-cause hospitalization among dual-eligible nursing home residents (≥65 years) during the 180 days after a new prescription for a first-generation (N=3,611) or a second-generation (N=46,293) antipsychotic. Propensity scores created from Medicare and Medicaid Analytic eXtract data were used to identify a matched cohort of equal numbers of users of each antipsychotic class (N=3,609). The Cox proportional model and the extended Cox hazard model were used to evaluate the risk of all-cause hospitalization in the matched cohort.
Results
The unadjusted rates of all-cause hospitalization were 21.3% and 30.5% among users of first- and second-generation antipsychotics, respectively. The Cox proportional model revealed a significant difference between the two classes in risk of all-cause hospitalization (hazard ratio [HR]=1.33, p<.001). There was no difference in hospitalization risk among users of first- versus second-generation antipsychotics within the first 20 days of use; however, the odds of hospitalization among users of first-generation antipsychotics increased by 58% after 20 days of use (HR=1.58, p<.001).
Conclusions
The study findings suggest that use of first- versus second-generation antipsychotics lasting more than 20 days is associated with a differential risk of all-cause hospitalization, possibly due to differential safety profiles of the two classes. Consequently, there is a need to monitor the use of antipsychotics by elderly patients, especially after a period of initial use.
Antipsychotic medications are commonly used in nursing homes for treatment and management of schizophrenia, behavioral problems of dementia, and other psychiatric disorders (1,2). Since their introduction in the 1950s, first-generation antipsychotics, such as haloperidol and thioridazine, formed the mainstay of treatment of psychoses for several decades.
However, use of these drugs was associated with extrapyramidal symptoms, tardive dyskinesia, sedation, and other adverse events (1). This led to the development of second-generation antipsychotics in the 1990s. Consequently, second-generation antipsychotic agents, such as olanzapine and risperidone, are preferred over first-generation antipsychotic agents because of their relatively superior side effect profiles (1–4). Recent studies suggest that in 2007, over one-fourth of elderly residents of nursing homes used antipsychotics, mainly second-generation agents, for an annual expenditure of $309 million (5).
Despite the perceived superiority of second-generation agents, recent large trials suggest that both classes have comparable safety and effectiveness profiles (6,7). In addition, second-generation antipsychotic use has been associated with serious adverse events, such as cardiometabolic dysfunction, diabetes mellitus, falls and fractures, and cerebrovascular and other cardiovascular events, which may require hospitalization (8–12). There are also black-box warnings regarding the use of both first- and second-generation antipsychotics, and both classes are associated with risk of death among elderly patients with dementia (13,14). In view of the growing concern with second-generation antipsychotic use, it is important to look at the overall health care impact of the two antipsychotic classes.
Only four studies have examined the association of risk of all-cause hospitalization and use of first- versus second-generation agents in diverse populations (15–18), and they provided inconclusive evidence. The only study that focused on residents of long-term care found no difference in hospitalization risk among residents using first- versus second-generation antipsychotics (17). However, none of the studies focused on residents who were enrolled in both Medicare and Medicaid, known as dual-eligible beneficiaries, who are poorer and less healthy than their elderly counterparts and comprise a high proportion of residents in long-term care (19–21). As a result, they have higher unmet needs for care compared with persons who are not dual eligible (22).
This study aimed to compare the risk of all-cause hospitalization associated with use of first- and second-generation antipsychotics in the elderly population, given that all-cause hospitalization provides a measure of overall drug safety. The study findings are intended to clarify the comparative safety profiles of the two drug classes; this information will be useful to physicians who prescribe antipsychotics in the vulnerable elderly population. In particular, we focused on the risk of all-cause hospitalization among dual-eligible nursing home residents. The findings may provide insight into the overall safety profiles of the two antipsychotic classes in this vulnerable and understudied population.
Methods
Data sources
The study used multistate, multiyear data for elderly beneficiaries with dual eligibility for Medicaid and Medicare services. Medicaid and Medicare data from four states (Texas, New York, California, and Florida) for the years 2001 to 2003 were analyzed. These states were selected because they have large sample sizes and provide data representative of the U.S. population. The Medicaid Analytic eXtract (MAX) data involved use of personal summary, prescription, inpatient, and long-term-care files (23). The personal summary file contains information about demographic characteristics and annual and monthly Medicaid and Medicare eligibility for dual-eligible recipients. The prescription drug file includes detailed information on prescription medications dispensed to beneficiaries. The inpatient file captures information on inpatient hospital stays for each recipient. The long-term-care file provides information on services provided for each recipient in long-term-care institutions, including nursing facilities and intermediate-care facilities.
The Medicare data consisted of beneficiary summary files, denominator files, and Medicare Provider Analysis and Review (MEDPAR) files (24). The denominator file includes detailed demographic and enrollment information for each Medicare-enrolled individual in a given year. The MEDPAR files contain information on inpatient stays in hospitals and skilled-nursing facilities. The data elements in the MEDPAR file include demographic information, clinical information, dates of services, and charges and reimbursement information. More information on data structure and layout of Medicare and Medicaid databases can be found elsewhere (23–25).
Study design and sample
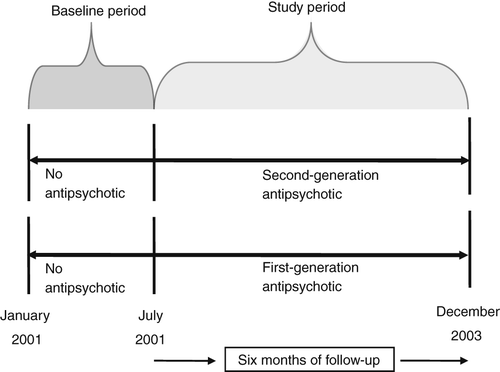
A retrospective, longitudinal cohort design with matched propensity scores was used to examine the risk of all-cause hospitalization among elderly nursing home residents receiving second-generation versus first-generation antipsychotics. Figure 1 provides a schematic presentation of the guidelines for selection of participants. The study population included all dual-eligible nursing home residents 65 years or older who initiated treatment with a first- or second-generation antipsychotic anytime between July 1, 2001, and December 31, 2003. Patients were identified as nursing home residents receiving antipsychotics if their index date fell anytime within the period of their nursing home stay. Only residents without a prescription for any of the above agents in the prior six months were included to protect against selection bias among prevalent users (26). Consistent with past research, the residents were followed for 180 days after the index date to examine the risk of all-cause hospitalization (27,28). The study was approved by the University of Houston Committee for the Protection of Human Subjects under exempt 4 category.
Exposure and outcome definitions
Exposure to first- or second-generation antipsychotics formed the primary independent variable of interest. The cohort of users of first-generation antipsychotics comprised residents who used any of the following agents: loxapine, fluphenazine, triflupromazine, chlorprothixene, haloperidol, chlorpromazine, thioridazine, promazine, trifluoperazine, thiothixene, molindone, perphenazine, acetophenazine, mesoridazine, pimozide, and perphenazine-amitriptyline. The cohort of users of second-generation antipsychotics consisted of residents who used clozapine, olanzapine, olanzapine-fluoxetine, risperidone, quetiapine, ziprasidone, or aripiprazole. Use of first- and second-generation antipsychotic agents was identified by corresponding National Drug Codes.
The primary outcome measure of this study was all-cause hospitalization within six months of antipsychotic treatment.
Propensity-score matching
The propensity score is defined as the probability of receiving a particular treatment given a set of observed covariates (29). Because antipsychotic treatment was not randomly assigned to the study population, the two treatment groups differed in terms of various observable and unobserved characteristics. The use of propensity scores aimed to balance the observed confounders across the users of first- and second-generation antipsychotics and make it possible to conduct analyses comparing the two groups.
A large number of covariates was included in the calculation of propensity score. The covariates were chosen on the basis of previously published literature, expert opinions, and evidence of their association with the treatment and the outcome (27,30). They included pretreatment characteristics, such as sociodemographic data (age, gender, and race), and clinical characteristics, such as comorbid conditions and other medications. Covariates related to health care utilization were measured during the six months before the index date. Severity of illness was also considered an important predictor of treatment allocation (16). All-cause hospitalization in the six months prior to the index date was used as a proxy for illness severity in this study.
A logistic regression model was developed by using the pretreatment characteristics as independent variables and receipt of first- and second-generation antipsychotics as the dependent variable to obtain propensity scores. Using the resulting predicted probabilities, we matched patients taking second-generation antipsychotics with patients taking first-generation antipsychotics by using a greedy matching technique (31). In this method, treated participants are first matched to participants in the control group on the first five digits of the propensity score. This process is repeated until the unmatched, treated participants are matched to their control group counterparts on the first digit of the propensity score. Participants that remain unmatched are excluded from analyses. Additional details on matching technique can be found elsewhere (32,33).
Statistical analysis
The differences in various pretreatment characteristics of the two groups before and after matching were evaluated by using chi square tests for categorical variables and t tests for continuous variables. Survival analysis was performed on the matched cohort to assess the risk of all-cause hospitalization among users of first-generation versus second-generation antipsychotics. Kaplan-Meier survival plots were created to depict the crude (unadjusted) relationship between antipsychotic use and time to hospitalization. Pairwise log-rank tests were used to compare survival curves for statistical difference (34,35). The Cox proportional-hazard (PH) model was used to examine risk of all-cause hospitalization within 180 days of initiating antipsychotic treatment. Patients were censored if the study period (180 days) ended without occurrence of the event, if they switched to a different antipsychotic class or discontinued the antipsychotic they had taken at initiation of therapy, if the gap between two successive refills of the same class of medication exceeded 30 days, or if they died, whichever occurred earlier. If a participant’s only claim for a particular antipsychotic agent occurred at the index date, the patient was included in the cohort until the length of supply of the prescribed medication.
A Cox PH regression model was stratified on matched pairs to examine the risk of all-cause hospitalization among users of first-generation versus second-generation antipsychotics, and corresponding hazard ratios (HRs) were obtained (34,35). Before using the Cox regression model, we checked the PH assumption for the model by including the interaction term between antipsychotic treatment (exposure) and log of time to hospitalization (outcome). The PH assumption for antipsychotic use was not met at the significance level of .05, indicating that the treatment effect was not constant over time. To adjust for time in our analysis, we used extended Cox models with a heaviside function. A cut point of 20 days was chosen on the basis of the intersection of the Kaplan-Meier curves for the two drug classes. All analyses were performed by using SAS, version 9.2.
Results
Matching process and patient characteristics
After applying inclusion and exclusion criteria, we identified 49,904 new users of antipsychotic agents (N=46,293 using second-generation antipsychotics; N=3,611 using first-generation antipsychotics). [A figure illustrating the identification process is available online as a data supplement to this article.] These antipsychotic users were used for calculation of propensity scores (c=.74). Data were missing for eight (.02%) residents on the age variable, and these were excluded from propensity-score calculation.
Table 1 reports baseline characteristics of the two groups before and after propensity-score matching. There were 7,218 users of antipsychotic agents in the final matched cohort, made up of equal numbers (N=3,609) of users of first-generation and second-generation antipsychotics. Figures 2 and 3 present the distribution of propensity scores before and after matching, respectively. The distributions of covariates before and after matching suggest that the two groups of antipsychotic users were comparable after matching.
| Unmatched (N=49,904) | Matched (N=7,218) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Secondgeneration(N=46,293) | Firstgeneration(N=3,611) | Secondgeneration(N=3,609) | Firstgeneration(N=3,609) | |||||||
| Characteristic | N | % | N | % | p | N | % | N | % | p |
| Age (M±SD) | 83.57±8.0 | 83.06±8.3 | <.001 | 82.97±8.3 | 83.05±8.3 | .71 | ||||
| Gender | <.001 | .98 | ||||||||
| Male | 13,048 | 28.2 | 1,267 | 35.1 | 1,267 | 35.1 | 1,266 | 35.1 | ||
| Female | 33,245 | 71.8 | 2,344 | 64.9 | 2,342 | 64.9 | 2,343 | 64.9 | ||
| Race | <.001 | .64 | ||||||||
| White | 31,207 | 67.4 | 2,264 | 62.7 | 2,223 | 61.6 | 2,262 | 62.7 | ||
| Black | 5,161 | 11.2 | 535 | 14.8 | 550 | 15.2 | 535 | 14.8 | ||
| Others | 9,925 | 21.4 | 812 | 22.5 | 836 | 23.2 | 812 | 22.5 | ||
| Hospitalization | <.001 | .86 | ||||||||
| Yes | 10,990 | 23.7 | 1,047 | 29.0 | 1,040 | 28.8 | 1,047 | 29.0 | ||
| No | 35,303 | 76.3 | 2,564 | 71.0 | 2,569 | 71.2 | 2,562 | 71.0 | ||
| Region | <.001 | |||||||||
| New York | 15,772 | 34.1 | 88 | 2.4 | 88 | 2.4 | 88 | 2.4 | ||
| Florida | 7,810 | 16.9 | 1,447 | 40.1 | 1,387 | 38.3 | 1,445 | 40.4 | ||
| California | 9,816 | 21.2 | 473 | 13.1 | 482 | 13.4 | 473 | 13.1 | ||
| Texas | 12,895 | 27.9 | 1,603 | 44.4 | 1,659 | 45.9 | 1,603 | 44.4 | ||
| Year of cohort entry | <.001 | .48 | ||||||||
| 2001 | 34,337 | 74.2 | 2,902 | 80.4 | 2,867 | 79.4 | 2,900 | 80.4 | ||
| 2002 | 10,963 | 23.7 | 647 | 17.9 | 669 | 18.5 | 647 | 17.9 | ||
| 2003 | 993 | 2.2 | 62 | 1.8 | 73 | 2.0 | 62 | 1.0 | ||
| Medical history | ||||||||||
| Hypertension | 10,660 | 23.0 | 1,061 | 29.4 | <.001 | 1,099 | 30.5 | 1,061 | 29.4 | .32 |
| Coronary heart disease | 3,832 | 8.3 | 321 | 8.9 | <.001 | 336 | 9.3 | 321 | 8.9 | .54 |
| Congestive heart failure | 6,373 | 13.8 | 668 | 18.5 | <.001 | 685 | 19.0 | 668 | 18.5 | .61 |
| Acute myocardial infarction | 708 | 1.5 | 76 | 2.1 | .007 | 75 | 2.1 | 76 | 2.1 | .93 |
| Cardiac arrhythmias | 3,258 | 7.0 | 305 | 8.5 | .001 | 302 | 8.4 | 305 | 8.5 | .97 |
| Circulatory disorder | 2,220 | 4.8 | 203 | 5.6 | .03 | 196 | 5.4 | 203 | 5.6 | .72 |
| Thromboembolic disorder | 569 | 1.2 | 47 | 1.3 | .70 | 41 | 1.1 | 47 | 1.3 | .52 |
| Diabetes | 5,541 | 12.0 | 535 | 14.8 | <.001 | 574 | 15.9 | 535 | 14.8 | .20 |
| Cerebrovascular disease | 5,356 | 11.6 | 589 | 16.3 | <.001 | 580 | 15.5 | 589 | 16.3 | .35 |
| Hip or femur fracture | 2,099 | 4.5 | 163 | 4.5 | .95 | 165 | 4.6 | 163 | 4.5 | .91 |
| Chronic obstructive pulmonary disorder | 3,766 | 8.1 | 382 | 10.6 | <.001 | 355 | 9.8 | 382 | 10.6 | .30 |
| Falls | 131 | .3 | 16 | .4 | .08 | 15 | .4 | 16 | .4 | .86 |
| Thyroid disorder | 4,841 | 10.1 | 482 | 13.4 | <.001 | 502 | 13.9 | 480 | 13.3 | .45 |
| Renal failure | 1,235 | 2.7 | 155 | 4.3 | <.001 | 164 | 4.5 | 155 | 4.3 | .60 |
| Other renal disorder | 4,970 | 10.7 | 468 | <.001 | 459 | 12.7 | 468 | 13.0 | .75 | |
| Liver disorder | 486 | 1.1 | 42 | 1.2 | .52 | 40 | 1.1 | 42 | 1.2 | .82 |
| Gastric disorder | 5,903 | 12.8 | 585 | 16.2 | <.001 | 557 | 15.4 | 585 | 16.2 | .37 |
| Ulcers | 1,748 | 3.8 | 171 | 4.7 | .004 | 180 | 5.0 | 171 | 4.7 | .62 |
| Cancer (any type) | 1,250 | 2.7 | 144 | 4.0 | <.001 | 146 | 4.1 | 144 | 4.0 | .90 |
| Cataract | 138 | .3 | 9 | .3 | .60 | 9 | .3 | 9 | .3 | .99 |
| Glaucoma | 525 | 1.1 | 44 | 1.2 | .65 | 48 | 1.3 | 44 | 1.2 | .68 |
| Anemia | 3,097 | 6.7 | 292 | 8.1 | .001 | 296 | 8.2 | 292 | 8.1 | .86 |
| Osteoporosis | 1,418 | 3.1 | 115 | 3.2 | .68 | 115 | 3.2 | 115 | 3.2 | .99 |
| Rheumatoid arthritis | 231 | .5 | 14 | .4 | .36 | 17 | .5 | 14 | .4 | .59 |
| Back pain | 628 | 1.4 | 47 | 1.3 | .79 | 45 | 1.3 | 47 | 1.3 | .83 |
| Dyslipidemia | 832 | 1.8 | 75 | 2.1 | .23 | 72 | 2.0 | 72 | 2.0 | .80 |
| Obesity | 188 | .4 | 21 | .6 | .12 | 21 | .6 | 21 | .6 | .99 |
| HIV infection | 21 | .1 | 2 | .1 | .68 | 4 | .1 | 2 | .1 | .68 |
| Pneumonia | 2,905 | 6.3 | 323 | 8.9 | <.001 | 308 | 8.5 | 323 | 8.9 | .53 |
| Parkinson’s disease | 1,212 | 2.6 | 82 | 2.3 | .21 | 86 | 2.4 | 82 | 2.3 | .75 |
| Endocarditis | 366 | .8 | 41 | 1.1 | .03 | 26 | .7 | 41 | 1.1 | .06 |
| Substance use disorder | 530 | 1.1 | 51 | 1.4 | .15 | 50 | 1.4 | 51 | 1.4 | .92 |
| Extrapyramidal symptoms | 103 | .2 | 13 | .4 | .10 | 8 | .2 | 13 | .4 | .27 |
| Dementia | 10,568 | 22.8 | 923 | 25.6 | <.001 | 968 | 26.8 | 923 | 25.6 | .23 |
| Schizophrenia | 2,447 | 5.3 | 206 | 5.7 | .28 | 210 | 5.8 | 206 | 5.7 | .84 |
| Anxiety disorder | 1,293 | 2.8 | 122 | 3.4 | .04 | 112 | 3.1 | 122 | 3.4 | .51 |
| Conduct disorder | 60 | .1 | 2 | .1 | .32 | 3 | .1 | 2 | .1 | .99 |
| Mood disorder | 4,377 | 9.5 | 319 | 8.8 | .22 | 321 | 8.9 | 319 | 8.8 | .93 |
| Other mental disorders | 805 | 1.7 | 86 | 2.4 | .005 | 89 | 2.4 | 86 | 2.4 | .82 |
| Other drugs used | ||||||||||
| Cardiovascular | 19,689 | 42.5 | 2,095 | 58.0 | <.001 | 2,119 | 58.7 | 2,094 | 58.0 | .55 |
| Antidiabetics | 6,399 | 13.8 | 737 | 20.4 | <.001 | 773 | 21.4 | 737 | 20.4 | .30 |
| Analgesic | 13,331 | 28.8 | 1,420 | 39.3 | <.001 | 1,414 | 39.2 | 1,419 | 39.3 | .90 |
| Estrogen | 1,679 | 3.6 | 150 | 4.2 | .10 | 139 | 3.9 | 150 | 4.2 | .51 |
| Antihistamine | 5,066 | 10.9 | 609 | 16.9 | <.001 | 588 | 16.3 | 609 | 16.9 | .51 |
| Antiasthmatics | 4,580 | 9.9 | 582 | 16.1 | <.001 | 549 | 15.2 | 582 | 16.1 | .29 |
| Anti-infective | 15,457 | 33.4 | 1,724 | 47.7 | <.001 | 1,786 | 49.5 | 1,723 | 47.7 | .14 |
| Diuretic | 10,961 | 23.7 | 1,183 | 32.8 | <.001 | 1,226 | 34.0 | 1,182 | 32.8 | .27 |
| Anticancer | 2,843 | 6.1 | 310 | 8.6 | <.001 | 319 | 8.8 | 310 | 8.6 | .71 |
| Anticholinergic | 2,407 | 5.2 | 332 | 9.2 | <.001 | 298 | 8.3 | 332 | 9.2 | .16 |
| Ophthalmic | 6,140 | 13.3 | 687 | 19.0 | <.001 | 697 | 19.3 | 686 | 19.0 | .74 |
| Antithyroid | 4,841 | 9.7 | 482 | 13.4 | <.001 | 502 | 13.9 | 480 | 13.3 | .45 |
| Antismoking | 23 | .1 | 1 | .0 | .99 | 3 | .1 | 1 | .0 | .62 |
| Endocrine and metabolic agent | 178 | .4 | 19 | .5 | .19 | 19 | .5 | 19 | .5 | .99 |
| Hypnotic | 4,788 | 10.3 | 471 | 13.0 | <.001 | 453 | 12.6 | 471 | 13.1 | .53 |
| Antidepressant | 14,631 | 31.6 | 1,264 | 35.0 | <.001 | 1,322 | 36.6 | 1,262 | 35.0 | .15 |
| Anticonvulsant | 5,955 | 12.9 | 539 | 14.9 | <.001 | 548 | 15.2 | 539 | 14.9 | .77 |
| Lithium | 105 | .2 | 7 | .2 | .69 | 10 | .3 | 7 | .2 | .46 |
| Antianxiety | 7,983 | 17.2 | 866 | 24.0 | <.001 | 885 | 24.5 | 865 | 24.0 | .58 |
| Stimulant | 131 | .3 | 6 | .2 | .25 | 12 | .3 | 6 | .2 | .24 |
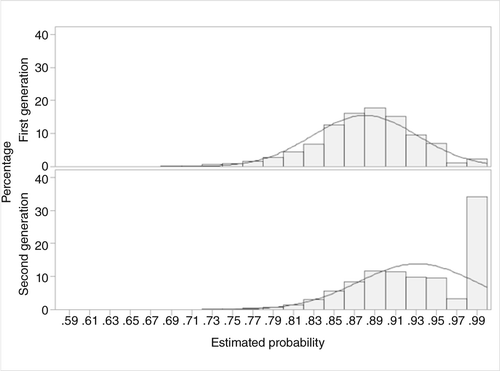
a Mean±SD propensity score=.88±.05, median propensity score=.88, for first-generation antipsychotics; mean±SD propensity score=.93±.06, median propensity score=.93, for second-generation antipsychotics. The curved line indicates normal distribution.
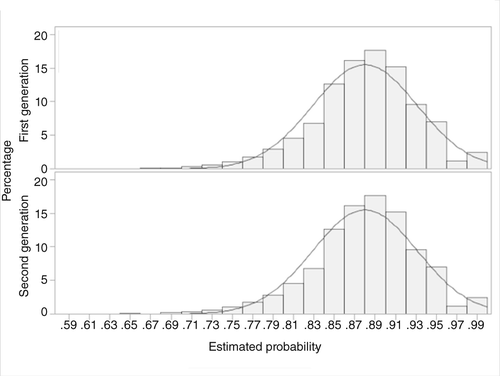
a Mean±SD propensity score=.88±.05, median propensity score=.88, first-generation antipsychotics; mean±SD=.88±.05, median=.88, second-generation antipsychotics. The curved line indicates normal distribution.
Risk of all-cause hospitalization
A total 1,869 individuals experienced all-cause hospitalization following the use of antipsychotic agents, including 1,101 (30.5%; 594.5 events per 100 patients per year) who used second-generation antipsychotics and 768 (21.3%; 616.6 events per 100 patients per year) who used first-generation antipsychotics. The median time to hospitalization was 49 days for users of second-generation antipsychotics and 46 days for users of first-generation antipsychotics (interquartile range 18–96 and 6–96 days, respectively). Figure 4 shows Kaplan-Meier survival curves evaluating hospitalization risk among elderly nursing home residents by antipsychotic use. The graph demonstrates a significant association between antipsychotic use and risk of all-cause hospitalization (p<.001).
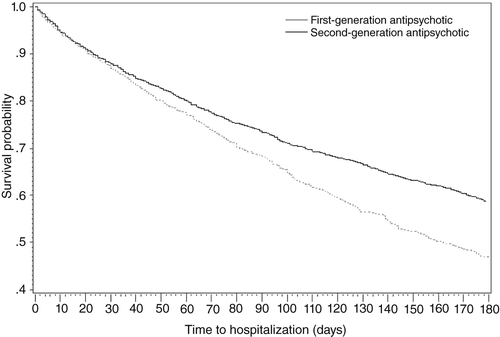
a Log-rank p<.001
The results of traditional Cox regression suggest that, on average, there was a significant difference in risk of all-cause hospitalization between first-generation and second-generation antipsychotic users (HR=1.33, 95% confidence interval [CI]=1.16–1.51, p<.001). The extended Cox model revealed that there was no difference in risk of hospitalization among users of first- versus second-generation antipsychotics within the first 20 days of use; however, among users of first-generation antipsychotics, the odds of hospitalization increased by 58% after 20 days of use (HR=1.58, CI=1.32–1.88, p<.001).
Discussion
This study examined the risk of all-cause hospitalization among dual-eligible elderly nursing home residents who were using antipsychotics and found that, on average, there was a significant difference in hospitalization risk among users of first-generation versus second-generation agents during the study period. However, as the extended Cox model revealed, the risk was similar only within the initial 20 days of use. After 20 days of first-generation drug use, there was a 58% increase in hospitalization risk.
This is the first study that has examined the risk of all-cause hospitalization associated with antipsychotic use among dual-eligible nursing home residents. The similarity in risk of hospitalization within the first 20 days of therapy with second- and first-generation agents does not indicate that there is no risk involved with antipsychotic use; rather, it suggests that both classes are associated with similar risk of hospitalization within the initial 20 days of therapy. The risk, however, increased 58% with extended use (between 20 and 180 days) of first-generation versus second-generation agents.
Previous observational studies in diverse health care settings found that use of second-generation agents is associated with a lower or equal risk of hospitalization compared with use of first-generation antipsychotics (15–18). However, none of the previous studies focused on dual-eligible residents, who are generally poorer and less healthy than their elderly counterparts and have higher unmet care needs (19–22). Simoni-Wastila and others (17) found that compared with nonuse of antipsychotics, use of antipsychotics had no significant effect on risk of hospitalization. This study involving dual-eligible residents found that the hospitalization risk associated with first- and second-generation antipsychotics was not constant over time. Instead, the comparative risk of all-cause hospitalization among users of first-generation antipsychotics increased moderately (33%). Overall, the difference in findings from this study and the literature can be explained by the difference in study populations and the operational definition of antipsychotic exposure.
The similar risk of hospitalization associated with first- and second-generation antipsychotics during the initial 20 days of therapy may be attributed to the drugs’ immediate effects on multifactorial receptor pathways, such as the dopamine receptor D2, the alpha-1 adrenergic receptor, and the 5-HT2A receptor. All antipsychotics block these receptors to some extent. The high risk of hospitalization associated with longer use of first-generation antipsychotics can be attributed to their differential and delayed effects involving multifactorial receptor pathways (36,37).
This study did not examine the specific reasons for hospitalization visits among participants in the study population. Future studies, therefore, are needed to better understand factors that contribute to the risk of antipsychotic-induced hospitalization. However, given the differential risk associated with short-term use (>20 days), there is a need to monitor elderly patients who use antipsychotics, especially after initial use. Consequently, clinicians should consider prescribing the lowest possible dose of antipsychotics for the shortest duration, whenever possible, and avoid concomitant prescription of other psychotropic medications that could predispose nursing-home residents to additional hospitalization risk.
The major strengths of this study were the study design and analytical approach. The propensity-matched, retrospective cohort design involved a large number of elderly, dual-eligible beneficiaries residing in nursing homes. The propensity-score model included a large number of potential confounders that could be related to antipsychotic treatment and risk of hospitalization. Only new users of antipsychotics were included in the study cohort to minimize prevalence bias (26). In addition, the study focused on dual-eligible nursing home residents, who represent one of the most understudied populations and have very high unmet need for long-term care (21).
The results should be viewed in light of the study’s limitations. The use of computer-recorded information to capture data did not allow us to ascertain whether the participants actually used their dispensed medicines. Identification of diseases and outcome measurements were based on diagnostic data in medical claims. Incomplete records submitted by providers, together with limited clinical details in the system, might affect the accuracy of administrative data. Variables included in the propensity score were limited to those available in the data source; thus unmeasured confounders, such as tobacco use, nutrition, health status, and cognitive and functional limitations, might have affected the study findings. These confounders, particularly limitations in cognitive and functional abilities, could lead to selective prescribing of first-generation antipsychotics to more vulnerable patients, possibly resulting in overestimation of the association between antipsychotic use and hospitalization risk (38). Future studies, including prospective studies, are needed to assess the issue of unobserved and residual confounding.
The study did not include newly available second-generation agents, such as asenapine and paliperidone, because of the study time frame. Future studies are needed to address the effects of other newly available second-generation agents in an effort to develop safety profiles of the entire antipsychotic class. Also, because of incomplete matching, results obtained from propensity-score matching can be applicable only to the final matched cohort of the population studied (39). All-cause hospitalization provides a generalized indication regarding the overall drug safety; future studies are needed to evaluate the specific reason for hospitalization among first-generation versus second-generation users. In addition, the study focused on the vulnerable dual-eligible beneficiaries; therefore, the findings may not be generalized to other population and settings.
Conclusions
Antipsychotic-induced adverse events generate a significant public health concern owing to their association with morbidity and mortality in the elderly. This retrospective cohort study that used matched propensity scores found that use of first-generation and second-generation antipsychotics was associated with a similar risk of all-cause hospitalization during the initial 20 days of therapy. The risk, however, increased (58%) with prolonged treatment with first-generation antipsychotics. The study findings suggest that multiple pharmacological mechanisms might play an important role in adverse events, which could lead to hospitalization. Given the comparable effectiveness profiles of first- and second-generation antipsychotic agents among the elderly (6), more safety research is needed to evaluate the specific reasons for hospitalization, and subsequent mortality, associated with antipsychotic use in nursing homes.
1 : A systematic review of atypical antipsychotic drugs in schizophrenia. Health Technology Assessment 7:1–193, 2003Crossref, Medline, Google Scholar
2 : Atypical antipsychotics in elderly patients with dementia or schizophrenia: review of recent literature. Harvard Review of Psychiatry 13:340–351, 2005Crossref, Medline, Google Scholar
3 : A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. Journal of the American Geriatrics Society 54:354–361, 2006Crossref, Medline, Google Scholar
4 : Using antipsychotic agents in older patients. Journal of Clinical Psychiatry 65(suppl 2):5–99, 2004Medline, Google Scholar
5 Medicare Atypical Antipsychotic Drug Claims for Elderly Nursing Home Residents. Pub no OEI-07-08-00150. Washington, DC, US Department of Health and Human Services, Office of Inspector General, May 2011. Available at oig.hhs.gov/oei/reports/oei-07-08-00150.pdfGoogle Scholar
6 : Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209–1223, 2005Crossref, Medline, Google Scholar
7 : Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Archives of General Psychiatry 63:1079–1087, 2006Crossref, Medline, Google Scholar
8 : Controversies in behavioral neurology: the use of atypical antipsychotic drugs to treat neurobehavioral symptoms in dementia. Current Neurology and Neuroscience Reports 8:471–474, 2008Crossref, Medline, Google Scholar
9 : Use of antipsychotics in elderly patients with dementia: do atypical and conventional agents have a similar safety profile? Pharmacological Research 59:1–12, 2009Crossref, Medline, Google Scholar
10 : Managing weight gain and metabolic issues in patients treated with atypical antipsychotics. Journal of Clinical Psychiatry 69:e04, 2008Crossref, Medline, Google Scholar
11 : Atypical antipsychotic drugs and diabetes mellitus in the US Food and Drug Administration adverse event database: a systematic Bayesian signal detection analysis. Psychopharmacology Bulletin 42:11–31, 2009Medline, Google Scholar
12 : Atypical antipsychotic drugs and the risk of sudden cardiac death. New England Journal of Medicine 360:225–235, 2009Crossref, Medline, Google Scholar
13 Public Health Advisory: Deaths With Antipsychotics in Elderly Patients With Behavioral Disturbances. Silver Spring, Md, US Food and Drug Administration, 2013. Available at www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm. Accessed Dec 3, 2010Google Scholar
14 Information for Healthcare Professionals: Conventional Antipsychotics. Silver Spring, Md, US Food and Drug Administration, 2013. Available at www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm124830.htm. Accessed Dec 3, 2010Google Scholar
15 : The association between class of antipsychotic and rates of hospitalization: results of a retrospective analysis of data from the 2005 Medicare current beneficiary survey. Clinical Therapeutics 31:2931–2939, 2009Crossref, Medline, Google Scholar
16 : Hospitalization risk associated with typical and atypical antipsychotic use in community-dwelling elderly patients. American Journal of Geriatric Pharmacotherapy 6:198–204, 2008Crossref, Medline, Google Scholar
17 : Association of antipsychotic use with hospital events and mortality among Medicare beneficiaries residing in long-term care facilities. American Journal of Geriatric Psychiatry 17:417–427, 2009Crossref, Medline, Google Scholar
18 : Analysis of healthcare utilization patterns and adherence in patients receiving typical and atypical antipsychotic medications. Current Medical Research Opinion 19:619–626, 2003Crossref, Medline, Google Scholar
19 : Prescription drug use and expenditures among dually eligible beneficiaries. Health Care Financing Review 28:43–56, 2007Medline, Google Scholar
20 Health Care Spending and the Medicare Program. Washington, DC, Medicare Payment Advisory Commission, June 2010. Available at www.medpac.gov/documents/Jun10DataBookEntireReport.pdfGoogle Scholar
21 Dual Eligibles: Medicaid Role in Filling the Gaps. Washington, DC, Kaiser Commission on Medicaid and Uninsured. Available at kaiserfamilyfoundation.files.wordpress.com/2013/01/dual-eligibles-medicaid-s-role-in-filling-medicare-s-gaps.pdf. Accessed Dec 3, 2010Google Scholar
22 : Unmet long-term care needs: an analysis of Medicare-Medicaid dual eligibles. Inquiry 42:171–182, 2005Crossref, Medline, Google Scholar
23 Medicaid Analytic eXtract (MAX) General Information. Baltimore, Centers for Medicare and Medicaid Services, Oct 2013. Available at www.cms.hhs.gov/MedicaidDataSourcesGenInfo/07_MAXGeneralInformation.asp. Accessed Dec 3, 2010Google Scholar
24 Standard Analytical Files. Baltimore, Centers for Medicare and Medicaid Services, Dec 2013. Available at www.cms.hhs.gov/IdentifiableDataFiles/02_StandardAnalyticalFiles.asp. Accessed Dec 3, 2010Google Scholar
25 Find a CMS Data File. Minneapolis, Minn, Research Data Assistance Center. Available at www.resdac.umn.edu/Available_CMS_Data.asp. Accessed Dec 3, 2010Google Scholar
26 : Evaluating medication effects outside of clinical trials: new-user designs. American Journal of Epidemiology 158:915–920, 2003Crossref, Medline, Google Scholar
27 : Risk of death in dual-eligible nursing home residents using typical or atypical antipsychotic agents. Medical Care 50:961–969, 2012Crossref, Medline, Google Scholar
28 : Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Canadian Medical Association Journal 176:627–632, 2007Crossref, Medline, Google Scholar
29 : The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55, 1983Crossref, Google Scholar
30 : Variable selection for propensity score models. American Journal of Epidemiology 163:1149–1156, 2006Crossref, Medline, Google Scholar
31 Parson LS: Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. Edmonds, Wash, Ovation Research Group, 2001. Available at www2.sas.com/proceedings/sugi26/p214-26.pdfGoogle Scholar
32 : Type I error rates, coverage of confidence intervals, and variance estimation in propensity-score matched analyses. International Journal of Biostatistics, 2009; doi 10.2202/1557-4679.1146Crossref, Medline, Google Scholar
33 Alexander MT, Kufera JA: Butting Heads on Matched Cohort Analysis Using SAS Software. Baltimore, University of Maryland School of Medicine, National Study Center for Trauma and Emergency Medical Systems, 2007. Available at www.nesug.org/proceedings/nesug07/sa/sa01.pdfGoogle Scholar
34 : Regression Methods in Biostatistics: Linear, Logistic, Survival and Repeated Measures Models. New York, Springer, 2005Google Scholar
35 : Survival Analysis: A Self-Learning Text. New York, Springer-Verlag, 1996Crossref, Google Scholar
36 : Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biological Psychiatry 50:873–883, 2001Crossref, Medline, Google Scholar
37 : Describing an atypical antipsychotic: receptor binding and its role in pathophysiology. Primary Care Companion to the Journal of Clinical Psychiatry 5:9–13, 2003Google Scholar
38 : Assessing residual confounding of the association between antipsychotic medications and risk of death using survey data. CNS Drugs 23:171–180, 2009Crossref, Medline, Google Scholar
39 : Estimating exposure effects by modelling the expectation of exposure conditional on confounders. Biometrics 48:479–495, 1992Crossref, Medline, Google Scholar



