The Definition and Prevalence of Pediatric Psychotropic Polypharmacy
Abstract
Objectives:
Using increasingly stringent criteria, this study evaluated the prevalence of psychotropic polypharmacy among children on the basis of duration of overlap between two or more psychotropic medications.
Methods:
The prevalence of psychotropic polypharmacy was defined as receiving ≥14 days, ≥30 days, ≥60 days, and ≥90 days of overlapping psychotropic prescription fills. Descriptive analysis was used to compare the prevalence findings on the basis of multistate Medicaid data involving children six to 18 years of age. A sensitivity analysis was also conducted to explore the extent to which the cross-sectional operational definitions of polypharmacy used in the published literature identified patients who were prescribed psychotropic combinations on a long-term basis.
Results:
The analysis revealed that 282,910 children had at least one psychotropic prescription fill in 2005. Of these patients, 28.8% received psychotropic combinations for at least 14 consecutive days. The observed rate of polypharmacy dropped to 27.2% with 30 days of overlap and to 20.9% with 60 days of overlap. Using a 60-day overlap in psychotropic drugs as a cutoff between short-term and long-term polypharmacy, analyses showed that 14%–46% of patients identified by cross-sectional definitions as receiving polypharmacy had likely received combination treatment on a temporary rather than on a long-term basis. In addition, cross-sectional definitions failed to identify 18%–44% of patients classified as receiving long-term polypharmacy (≥60-day overlap).
Conclusions:
The observed rate of polypharmacy dropped with increasingly stringent operational definitions for polypharmacy. The findings suggest that considerable differences arise when comparing rates of polypharmacy across studies with inconsistent operational definitions. (Psychiatric Services 62:1450–1455, 2011)
The use of combinations of psychotropic medications (polypharmacy) is on the rise among children and adolescents. The prevalence of this practice has increased two- to sevenfold in the past decade (1,2). The increase in use of psychotropic polypharmacy has heightened public concern because, aside from studies of stimulants, few controlled studies exist concerning the efficacy and safety of psychotropic monotherapy for children and adolescents, let alone that of psychotropic polypharmacy (3).
Despite the paucity of evidence for or against polypharmacy, in some situations short-term polypharmacy is clearly appropriate or even necessary. For instance, when changing a patient from one psychotropic drug to another, most clinicians choose to overlap or cross-titrate the two, resulting in a purposely brief period of combination treatment (4–6). Other clinicians, especially in the inpatient setting, often view second-generation antipsychotics as temporarily useful adjuncts to stimulants or mood stabilizers in treating exacerbations of mental illness. In each of these instances, the long-term goal is still monotherapy, but combinations are used to achieve short-term goals, such as to reduce risks while switching medications or to promote a more rapid resolution of acute symptoms.
Although the empirical base for short-term use of psychotropic combinations in the pediatric population is not particularly strong, the rationale is grounded in clinical experience and involves relatively brief exposure to the risks of the combination. On the other hand, long-term maintenance treatment with psychotropic combinations raises far more significant questions about efficacy, safety, and costs. Few data from clinical trials and observational studies are available to clarify the long-term effects of psychotropic combinations for children and adolescents.
From 1994 to 2009, a total of 13 studies specifically reviewed rates of polypharmacy in the pediatric population (2,7–18). The estimated prevalence of psychotropic polypharmacy ranged from 13.6% among children insured by Medicaid and who filled at least one psychotropic prescription (12) to 72% among youths in foster care (2).
Despite the variations in age groups, study designs, data sources, and clinical settings, most of these studies were cross-sectional evaluations of the prescribing of psychotropic medication at a single point in time or during a specific time frame, such as at hospital discharge or within the same month- or year-long period (1,2,7–13). The overlapping period of concurrent use often was not considered in the definitions; therefore, these studies may not have captured polypharmacy. First, there may not have been actual overlap of medications prescribed during the same month or the same year. Second, the proportion of patients who had only temporary overlap of psychotropic medications and were receiving necessary treatment (such as overlap during the cross-taper period) versus the proportion who were receiving persistent, long-term polypharmacy is unknown.
A few studies used data from adults (19–23) and defined polypharmacy as concurrent, overlapping treatment with multiple psychotropic agents for a specified duration (at least 30 days, up to 90 days, and so on). A 60-day overlap period has been the most commonly used criterion in the research literature for indicating long-term polypharmacy.
Therefore, the first objective of this study was to evaluate psychotropic polypharmacy by using an increasingly stringent operational definition based on days of overlapping medication supplies (≥14, ≥30, ≥ 60, and ≥90 days). The second objective was to compare the prevalence of polypharmacy obtained on the basis of the 60-day overlap criterion with prevalence derived from several cross-sectional operational definitions of polypharmacy used in published pediatric studies.
Methods
Data source
The 2005 Medicaid Analytic eXtract (MAX) files created by the Centers for Medicare and Medicaid Services (CMS) were used to validate the definition of long-term psychotropic polypharmacy among children. MAX is a set of person-level data files containing information on Medicaid eligibility, service utilization, and payments. It is extracted from medical and pharmacy claims collected from the 50 state Medicaid programs. In our analysis, only Medicaid eligibility and pharmacy claims were used. Because it is difficult to analyze data from all 50 states, we used data from the four states with the largest Medicaid enrollment of children and adolescents (California, Illinois, New York, and Texas).
Psychotropic medications
Six psychotropic medication groupings included drugs for attention-deficit hyperactivity disorder, such as stimulants or atomoxetine; antidepressants (selective serotonin reuptake inhibitors, selective serotonin norepinephrine reuptake inhibitors, serotonin modulators, and tricyclic antidepressants); antipsychotic agents (first- and second-generation agents); lithium; anticonvulsant mood stabilizers (such as divalproex, oxcarbazepine, topiramate, and carbamazepine); and antianxiety drugs (such as hydroxyzine and benzodiazepines) (2). Alpha-agonists and antiparkinsonism medications were excluded from the study (17).
Study sample
The study sample consisted of all children and adolescents ages six to 18 who were enrolled in the California, Illinois, New York, or Texas Medicaid program in 2005 and filled at least one psychotropic medication prescription during the observation period. Because anticonvulsants are indicated for both epilepsy and mood disorders, patients with a diagnosis of epilepsy were excluded from this analysis.
Measures
The prevalence of psychotropic polypharmacy was determined by using increasingly stringent criteria for the duration of overlap between two or more psychotropic medications either from the same or different therapeutic categories (22). Specifically, the prevalence of combination treatments was defined as receiving ≥14, ≥30, ≥60, and ≥90 consecutive days of overlapping psychotropic prescription fills, and the findings were compared.
When defining periods of concurrent psychotropic coverage, we first determined the coverage period for each psychotropic medication. Prescription fills on the same day for different medications were assumed to be used concurrently in order to provide a desired medication regimen. Fills for the same medication that occurred on different days were assumed to be for serial use; any overlaps in supply were carried forward on the assumption that patients used all of the medications they received.
Because this study focused on only one year of data (2005), it is possible that a short-term polypharmacy episode observed at the beginning of 2005 was actually long-term use initiated in 2004. Similarly, a polypharmacy episode occurring at the end of 2005 could have continued into 2006. In order to ensure that all short-term and long-term uses were correctly classified, we excluded data from polypharmacy recipients whose polypharmacy episodes occurred in January 2005 and who had the same prescriptions filled in December 2004. For a similar reason, data from patients whose polypharmacy episodes occurred after October 1, 2005, were also were excluded.
The prevalence of psychotropic polypharmacy also was estimated with the cross-sectional operational definitions of polypharmacy from previous pediatric studies. These definitions are summarized in Table 1.
A sensitivity analysis was conducted to further explore the extent to which the polypharmacy definitions used in the literature identified patients who were prescribed psychotropic combinations on a long-term basis. First, using the 60-day overlap criterion as the standard for long-term treatment, we first calculated for each polypharmacy definition from the literature the number of “true positives,” or the number of patients classified as being prescribed polypharmacy by the 60-day overlap criterion who were similarly classified by the definition used in the literature. This cutoff was selected for several reasons. First, it is the most commonly used cutoff to define long-term polypharmacy in studies of adults (19,22–25). Second, the cross-titration of psychotropic medications typically takes two to three weeks, although in special cases an extended cross-titration period of around two months is necessary (4–6). And third, empirical studies supporting the use of psychotropic combinations with pediatric patients often followed patients for a limited period (eight weeks or less). The long-term effect beyond this cutoff is largely unknown.
We also calculated the number of “false positives,” or the number of patients classified as having been prescribed polypharmacy by the cross-sectional definition but not by the 60-day overlap criterion. In this study, such cases likely represent patients who were prescribed polypharmacy on a short-term, temporary basis. We also calculated the number of “false negatives,” representing patients who were classified as having been prescribed polypharmacy by the 60-day overlap criterion yet were not identified by the cross-sectional definition. In this study, this likely represents patients who were missed by cross-sectional definitions that focused on very narrow observation windows (seven days, for example). Finally, we calculated the sensitivity of each cross-sectional operational definition by dividing the number of true positives by the sum of true positives plus false negatives.
Results
Prevalence of polypharmacy by stringency of overlap criteria
A total of 511,034 patients between ages six and 18 filled at least one psychotropic prescription in 2005. Of these patients, 344,124 had 12 months of continuous Medicaid eligibility. After removing data for 30,507 patients with epilepsy, 29,412 patients with polypharmacy episodes that occurred after October 1, 2005, and 1,295 who continued their polypharmacy episodes from 2004, our final cohort consisted of 282,910 patients (Table 2). Of these, 28.8% received psychotropic combinations for at least 14 consecutive days. The observed rate of polypharmacy dropped to 27.2% when the overlap criterion was increased to 30 days and to 20.9% when the criterion was increased to 60 days of overlapping treatment. The prevalence of polypharmacy was only 17.7% when a minimum of 90 days of treatment overlap was required.
By using 60 days of treatment overlap as a cutoff between long-term and short-term use, we found that approximately a quarter (23%–27%) of patients who were classified as having been prescribed a polypharmacy regimen according to less stringent criteria (14-day or 30-day overlap) were likely receiving two or more psychotropic agents on only a temporary rather than on a long-term basis.
Of the 59,258 long-term psychotropic polypharmacy recipients, a vast majority (N=55,611; 93.8%) received combinations of psychotropic drugs from different therapeutic classes. The most commonly used combinations were antipsychotics with stimulants (N=24,361) and antidepressants with stimulants (N=16,780), followed by antidepressants combined with antipsychotics (N=15,183)and antipsychotics with anticonvulsants (N=13,015), and a patient could have received more than one combination during the study period.
Prevalence of polypharmacy based on cross-sectional definitions
Table 2 shows how the rates of polypharmacy derived from cross-sectional definitions used in previous studies differ from the rates obtained with the 60-day overlap criterion. The prevalence ranged from 17.9%, according to a definition of polypharmacy as having filled a prescription from a different therapeutic class within seven days of the index fill, to 39.1%, according to the definition that only required receipt of two or more psychotropic drugs within the one-year observation period. The analyses revealed that 14%–46% of classifications of polypharmacy according to cross-sectional definitions were false positives, meaning that the patients either received combinations for only short periods or never received those medications concurrently. In addition, cross-sectional definitions failed to identify 18% to 44% of patients classified as being prescribed long-term polypharmacy (≥60-day overlap).
Discussion
This study characterizes one of the first confirmatory findings of psychotropic polypharmacy on a continuum of most stringent to least stringent criteria for examining treatment overlap among children and adolescents covered by Medicaid. As expected, the prevalence of polypharmacy decreased with increasing stringency of the overlap criteria. On the basis of the 14-day overlap criterion, more than one-quarter (28.8%) of children and adolescents received psychotropic polypharmacy. Of these patients, 72.7% had been using the combinations for at least 60 days, and 61.3% took the combinations for 90 days or longer. According to the long-term polypharmacy criterion (≥60 days) operationally defined in our study, a majority of children and adolescents insured by Medicaid who received psychotropic combinations were taking these medications over an extended period.
The term “polypharmacy” does not in itself suggest whether its use is warranted. There are certainly some well-researched uses of medication combinations. For instance, emerging empirical data from adolescent studies show that the addition of a second-generation antipsychotic to a mood stabilizer may decrease bipolar disorder symptoms and improve overall response rates compared with monotherapy (26,27). These trials, however, followed patients for a limited period (six to eight weeks). The long-term efficacy and safety of these combination treatments remain unknown. Considering that there are no data from either clinical trials or observational studies to clarify the efficacy, safety, and tolerability of long-term treatment with psychotropic combinations in the pediatric population, long-term maintenance combination treatment raises far more significant questions compared with short-term combination treatment.
The appropriateness of using psychotropic polypharmacy is also dependent on individual characteristics such as age. In pediatric psychiatry, because of the relatively rich knowledge concerning use of psychotropic drugs with children in the older age groups, polypharmacy tends to be more acceptable with increasing patient age. Similarly, using three or more psychotropic medications concurrently is often seen as a questionable practice. Only a few studies have assessed extent of the use of potentially inappropriate polypharmacy in the pediatric population. A recent study by Essock and colleagues (28) reported that 13% of children were prescribed three or more concurrent psychotropic drugs for 90 days or longer in New York's Medicaid program. Future study is needed to confirm whether these questionable practices lead to increased adverse outcomes.
In addition to estimating prevalence of polypharmacy by using increasingly stringent criteria concerning medication overlap, we also examined the accuracy of alternative criteria for identifying patients receiving long-term, persistent combination psychotropic treatment. Although the prevalence rates of psychotropic polypharmacy estimated on the basis of some cross-sectional definitions were similar to what was estimated with the 60-day overlap criterion (22.4% versus 20.9%), the cross-sectional definitions often identified entirely different patients.
Operational definitions of polypharmacy that are cross-sectional or that examine prescribing patterns within a narrow time window both miss patients who are prescribed long-term combination treatments and include patients who are receiving only short-term overlapping prescriptions. For instance, one definition used in a previous study defined psychotropic polypharmacy as filling a prescription from a different therapeutic class within seven days of the index fill (12). Our analysis showed that the definition failed to identify one-third of long-term polypharmacy recipients because these patients received treatment augmentation beyond the seven-day window (a false-negative rate of 34.0%). On the other hand, many patients identified as receiving psychotropic polypharmacy on the basis of operational criteria from previous cross-sectional studies did not have long-term, persistent use of combination treatments.
Several previous studies defined polypharmacy as receiving two or more psychotropic drugs within a month or a year. Because an overlap between prescriptions was not required with the definitions used in those studies, it is likely that some patients never used the medications concurrently or the brief overlap due to cross-titration was considered polypharmacy. Sensitivity analysis showed that 14% to 46% of the patients identified in these cross-sectional definitions were not using polypharmacy on a long-term basis. A similar problem was observed in the studies that defined polypharmacy as receiving two or more psychotropic drugs with at least one day of overlap—33.0% of patients identified according to this definition received psychotropic combinations for only a limited period (<60 days).
The comparison between our observations and previous studies illustrates the considerable problems that arise when comparing rates of polypharmacy across studies with inconsistent operational definitions. In previous studies that reviewed rates of polypharmacy in the pediatric population (2,10–16), the estimated prevalence for psychotropic polypharmacy was the lowest (13.6%) for children receiving Medicaid (12). It remains unknown whether the use of psychotropic polypharmacy is lower among children and adolescents receiving Medicaid compared with those in other treatment settings or whether the differences in prevalence observed across studies can be explained entirely by the varying operational definitions used. This question merits further exploration.
The main limitation of the study is that data on dispensed prescriptions do not necessarily reflect actual consumption. Our use of administrative encounter data in this study also precluded any efforts to determine, from the perspective of the patient or the provider, reasons for the prescription of multiple psychotropic agents. One of the theoretical rationales supporting long-term polypharmacy is the use of multiple medications with different mechanisms of action, with the hope that this will address several areas of symptomatology of patients with comorbid psychiatric disorders. Another theoretical rationale for the use of multiple agents is a relative avoidance of side effects. In mental disorders that require long-term maintenance such as postconcussional disorder or bipolar disorder, use of several agents in combination, each below their individual side effect threshold (and below that for the combination), may more readily avoid side effects than using a single drug (29,30).
Conclusions
The observed rate of polypharmacy dropped with increasingly stringent operational definitions of polypharmacy. The findings suggest that considerable differences arise when comparing rates of polypharmacy across studies with inconsistent operational definitions. Additional studies are needed to more fully appreciate the utilization pattern of long-term psychotropic polypharmacy, the clinical reasons for its use, and the outcomes of this treatment strategy when used with children and adolescents with different mental disorders.
1 : Polypharmacy in children and adolescents treated for major depressive disorder: a claims database study. Journal of Clinical Psychiatry 70:240–246, 2009 Crossref, Medline, Google Scholar
2 : Psychotropic medication patterns among youth in foster care. Pediatrics 121:157–163, 2008 Crossref, Medline, Google Scholar
3 : Psychiatric polypharmacy and children: examining pediatric multipsychotropic regimens. Behavioral Health Management, Sept 1, 2004. Available at www.allbusiness.com/health-care-social-assistance/256621-1.html. Accessed
4 : The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry 8:95, 2008 Crossref, Medline, Google Scholar
5 : Transition from methylphenidate or amphetamine to atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder—a preliminary tolerability and efficacy study. Clinical Therapeutics 29:1168–1177, 2007 Crossref, Medline, Google Scholar
6 Psychotropic Medication Utilization Parameters for Children in State Custody. Austin, Texas Department of State Health Services. Available at www.state.tn.us/youth/dcsguide/policies/chap20/PsychoMedUtilGuide.pdf Google Scholar
7 : Multiple psychotropic medication use for youths: a two-state comparison. Journal of Child and Adolescent Psychopharmacology 15:68–77, 2005 Crossref, Medline, Google Scholar
8 : Concomitant pharmacotherapy among youths treated in routine psychiatric practice. Journal of Child and Adolescent Psychopharmacology 15:12–25, 2005 Crossref, Medline, Google Scholar
9 : Psychotropic co-medication among stimulant-treated children in the Netherlands. Journal of Child and Adolescent Psychopharmacology 15:38–43, 2005 Crossref, Medline, Google Scholar
10 : Predictive factors for polypharmacy among child and adolescent psychiatry inpatients. Clinical Practice and Epidemiology in Mental Health 2:25, 2006 Crossref, Google Scholar
11 : Psychotropic prescribing practices in child psychiatric inpatients 9 years old and younger. Journal of Child and Adolescent Psychopharmacology 14:95–103, 2004 Crossref, Medline, Google Scholar
12 : Multiple psychotropic pharmacotherapy among children and adolescent enrollees in Connecticut Medicaid managed care. Psychiatric Services 54:72–77, 2003 Link, Google Scholar
13 : Psychotropic medication in child and adolescent mental health service. Journal of Child and Adolescent Psychopharmacology 16:273–285, 2006 Crossref, Medline, Google Scholar
14 : Prescribing practices of outpatient child psychiatrists. Journal of the American Academy of Child and Adolescent Psychiatry 33:35–44, 1994 Crossref, Medline, Google Scholar
15 : Trends in combined pharmacotherapy with stimulants for children. Psychiatric Services 53:244, 2002 Link, Google Scholar
16 : Changing patterns of psychotropic medications prescribed by child psychiatrists in the 1990s. Journal of Child and Adolescent Psychopharmacology 7:267–274, 1997 Crossref, Medline, Google Scholar
17 : State variation in psychotropic medication use by foster care children with autism spectrum disorder. Pediatrics 124:305–312, 2009 Crossref, Medline, Google Scholar
18 : Psychotropic medication use among Medicaid enrolled children with autism spectrum disorders. Pediatrics 121:441–448, 2008 Crossref, Medline, Google Scholar
19 : Prevalence, trends, and factors associated with antipsychotic polypharmacy among Medicaid eligible schizophrenia patients, 1998–2000. Journal of Clinical Psychiatry 65:1377–1388, 2004 Crossref, Medline, Google Scholar
20 : Antipsychotic medication coprescribing in a large state hospital system. Pharmacoepidemiology and Drug Safety 12:41–48, 2003 Crossref, Medline, Google Scholar
21 : Combination antipsychotic therapy in clinical practice. Psychiatric Services 54:55–59, 2003 Link, Google Scholar
22 : Long-term combination antipsychotic treatment in VA patients with schizophrenia. Schizophrenia Research 84:90–99, 2006 Crossref, Medline, Google Scholar
23 : Long-term antipsychotic polypharmacy in the VA health system: patient characteristics and treatment patterns. Psychiatric Services 58:489–495, 2007 Link, Google Scholar
24 : Prevalence, utilization patterns, and predictors of antipsychotic polypharmacy: experience in a multistate Medicaid population, 1998–2003. Clinical Therapeutics 29:183–195, 2007 Crossref, Medline, Google Scholar
25 : The development of polypharmacy: a longitudinal study. Family Practice 17:261–267, 2000 Crossref, Medline, Google Scholar
26 : A double-blind, randomized placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. Journal of the American Academy of Child and Adolescent Psychiatry 41:1216–1223, 2002 Crossref, Medline, Google Scholar
27 : Open-label prospective trial of risperidone in combination with lithium or divalproex sodium in pediatric mania. Journal of Affective Disorders 82(suppl):S103–S111, 2004 Crossref, Medline, Google Scholar
28 : Identifying clinically questionable psychotropic prescribing practices for Medicaid recipients in New York State. Psychiatric Services 60:1595–1602, 2009 Link, Google Scholar
29 : Polypharmacy in Psychiatry. New York, Marcel Dekker, 2001 Google Scholar
30 : Monotherapy vs polypharmacy. Medscape Psychiatry 9, 2004. Available at cme.medscape.com/viewarticle/468952. Accessed
Figures and Tables

Table 1 Cross-sectional studies of prevalence of psychotropic polypharmacy among children and adolescents
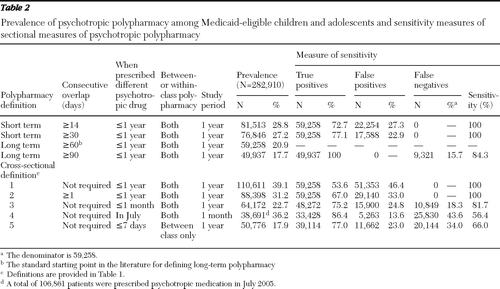
Table 2 Prevalence of psychotropic polypharmacy among Medicaid-eligible children and adolescents and sensitivity measures of sectional measures of psychotropic polypharmacy



