Alzheimer's Disease and Its Management in the Year 2010
Abstract
By 2010 the number of cases of Alzheimer's disease and other dementias will have increased by at least 25 percent. Alzheimer's disease poses an enormous threat to health service and public health resources. Three areas that require targeted research are early detection and recognition, biological markers and diagnosis, and pharmacotherapy. Care of patients with Alzheimer's disease in managed care organizations must be improved through a combination of research, education, advocacy, and legislation. Research in the pathogenic cascade of events within the brain leading to Alzheimer's disease has advanced, and treatment targets within the steps of the process have been specified. The current drug discovery and testing infrastructure is insufficient for major advances in therapy by 2010. Strategies for achieving an optimum outcome by then include increased funding of targeted research; expansion of drug discovery and therapeutic testing efforts; increased training of basic, clinical, and translational scientists; and study of optimum health care delivery systems.
Alzheimer's disease is an age-related disorder that becomes more common among increasingly elderly individuals. The size of the elderly population in the United States and the world is increasing, and the number of patients suffering from Alzheimer's disease and other dementing disorders will increase unless we find an intervention that prevents, delays the onset, slows the progression, or relieves the symptoms of these diseases. The cost of caring for cognitively impaired patients is enormous, and it will increase to imponderable proportions if better treatment and improved management are not identified.
This review of the prospects for the future management of Alzheimer's disease presents demographic projections to the year 2010, discusses factors that may modify the prevalence of dementia, emphasizes three areas in which additional research is needed—detection of dementia, biological markers of Alzheimer's disease, and treatment—and presents strategies for responding to the challenges posed by the growing population of Americans with dementia.
Prevalence of dementia in 2010
The population of the United States is changing rapidly, with an increased number and proportion of elderly individuals. In 1940 6.8 percent of the population was over age 65—about 9 million people. By 1960 this figure was 9.2 percent, or 16.6 million people. By 1980 the elderly population increased to 11.3 percent of the population, or 25.6 million people, and by 1990 to 12.5 percent, or 31.1 million. By 2010 a total of 13.3 percent of the population—39.7 million people—will be over age 65 (1).
The proportion of individuals over age 85 is increasing disproportionately rapidly. In 1940 only 4 percent, or 225,000 persons, were age 85 or older. This proportion increased to 5.6 percent, or 900,000, by 1960; to 8.8 percent, or 2.2 million, by 1980; and to 9.7 percent, or 3 million, by 1990. By 2010, overall 14.4 percent of the population—5.7 million individuals—will be over age 85 (1).
The increase in the number of elderly persons is accompanied by an increase in the prevalence of dementia, particularly Alzheimer's disease. The prevalence of dementia doubles every five years after the age of 60. One percent of those age 60 to 64 are affected, 2 percent of those age 65 to 69, 4 percent of those age 70 to 74, 8 percent of those age 75 to 79, 16 percent of those age 80 to 84, and 30 to 45 percent of those age 85 and older (2,3). Most of the cases of dementia are attributable to Alzheimer's disease.
These figures provide a reasonable guide to the number of patients with Alzheimer's disease and dementia anticipated in the year 2010, although several factors may affect these predictions, and changes in the constituency of the population with Alzheimer's disease can be anticipated. The increased educational level of the baby-boomer cohort will have the effect of delaying the age of appearance of symptoms of Alzheimer's disease, dissemination of dementia-related information will result in improved detection of dementia and an increase in the number of recognized early-stage patients, and administration of disease-modifying agents such as vitamin E will increase the number of patients in the middle phases of the illness. Also, improvements in general health and health care techniques will lengthen the survival of patients with dementia, increasing the number of severely affected patients and raising the level of medical comorbidity among patients with dementia.
Increased education is correlated with a lower frequency of Alzheimer's disease (4,5,6). The percentage of persons currently 75 years of age and older—those most vulnerable to Alzheimer's disease—who have at least a high school education is 58.7. Of those currently age 60 to 64 who will enter this period of maximum vulnerability by the year 2010, overall 75.5 percent have at least a high school education. A higher educational level among the at-risk cohort is expected to delay the onset of Alzheimer's disease and could decrease the overall prevalence by increasing the number of individuals who succumb to competing causes of mortality. This influence may be balanced or overtaken by overall greater longevity, longer survival of affected individuals, and advances in treating other causes of death in the elderly population.
Advances in the early detection of dementia will increase the number of patients recognized in milder stages of the illness. These individuals will present different management challenges than patients in more advanced phases of the disease. Issues related to employment, financial planning, driving, home care, and day care will be more pressing in this group.
The effects of therapy will have an impact on the composition of the population with Alzheimer's disease. Studies have shown that vitamin E slows the progress to defined milestones in the course (7), and treatment with cholinesterase inhibitors retards the rate of symptomatic cognitive decline (8,9,10). These agents have the effect of keeping individuals at more functional levels for longer periods of time. These treatments may increase the duration of the mild to moderate stages of the disease and increase the number of people with Alzheimer's disease who live in the community. Resources to support this population and their caregivers will be needed.
Early detection
Advances in therapies that slow the progression of Alzheimer's disease or improve existing symptoms will be most efficacious if applied early in the clinical course. Thus early detection is increasingly imperative and must be achieved if the emerging therapies described in this paper are to be used to greatest advantage. Besides facilitating early pharmacotherapy, recognition of mild dementia has other benefits that improve the care of patients and the well-being of their families. Early detection will make it easier for clinicians to identify sources of excess disability such as depression. It will allow differential diagnosis and identification of possible reversible causes of dementia such as hypothyroidism. Instituting nonpharmacologic interventions in early stages of the disease may reduce depression and anxiety, helping patients' families adjust to and plan for the future.
When Alzheimer's disease is detected in its early stages, patients can be more involved in decisions about competency and advanced directives at a time when they are more able to appreciate the consequences of their decisions. Patients and their families can also address issues affecting patient safety and vulnerability such as driving, making financial decisions, and being responsible for grandchildren and other family members.
Alzheimer's disease and other dementias are currently underrecognized. In the United Kingdom, O'Connor and colleagues (11) found that general practitioners recognized only 58 percent of patients with Alzheimer's disease who were identified by research psychiatrists using a structured diagnostic interview. In a U.S. study, Callahan and associates (12) found that only 3.2 percent of patients with mild cognitive impairment were recognized by general practitioners as being intellectually compromised, and only 23.5 percent of those with moderate to severe dementia were identified as having a dementia syndrome.
Efforts to improve recognition of dementia by 2010 must take into account the increasing influence of managed care organizations in health services delivery. Increasingly, Medicare beneficiaries who constitute the at-risk group for dementia receive services through plans with health maintenance organizations (HMOs). Managed care organizations provide a structure through which physicians can be more readily educated and practice can be standardized through management guidelines and care pathways. Such characteristics of HMOs can be used to improve the care of patients with Alzheimer's disease and their families.
On the other hand, cost containment pressures may decrease the time that health care professionals have available for each patient and militate against including the caregiver in patient interviews and in care planning. In addition, such pressures may provide overt or covert disincentives for detecting mild disorders when detection may have no documented cost benefit. The way in which these issues are resolved will affect the quality of care received by patients with dementia in managed care organizations.
Several strategies may facilitate the early recognition of dementia syndromes. Education is necessary to promote increased recognition by families and general practitioners. This approach might involve broad-based awareness-raising efforts, with public service announcements, media campaigns, and health fairs to increase the general public's knowledge about the diagnostic implications of memory loss in elderly persons. Pharmaceutical companies with direct-to-consumer advertising will further enhance consumer interest. Translational research, or research aimed at discovering the best means of translating scientific advances into improved clinical care, will be needed to define the best means of promoting detection of dementia in general practices. Advocacy by Medicare beneficiaries for dementia-related services from managed care organizations may improve the responsiveness of these organizations to patients with dementia and their caregivers. Legislation could modify Medicare contracting with managed care organizations to require dementia screening and dementia-related services.
Diagnosis and identification of biological markers
Currently, diagnosis of Alzheimer's disease depends on recognition of the characteristic clinical features. Clinically, diagnoses of Alzheimer's disease as defined by research criteria (13) are confirmed by autopsy to be 85 to 90 percent accurate (14). An inclusionary diagnosis of Alzheimer's disease is supported by identifying a core set of clinical features and bolstered by excluding other common causes of dementia in elderly persons.
The current approach to diagnosing Alzheimer's disease is time-intensive, labor-intensive, and costly. Its sensitivity and specificity are largely dependent on the expertise of the examiner. The discovery of biological markers would greatly improve the current approach to diagnosis. Except for rare autosomal dominant mutations, no biological markers for Alzheimer's disease are available before biopsy- or autopsy-based histological verification of the clinical diagnosis is obtained.
Discovery of a biological marker of Alzheimer's disease might allow a definite diagnosis before autopsy, facilitate early detection, and improve differential diagnosis of Alzheimer's disease and recognition of dementia syndromes that are not Alzheimer's disease. Such a discovery would also provide an additional marker for treatment response, improve identification of Alzheimer's disease among patients with mixed dementia syndromes consisting of Alzheimer's disease plus a second brain disorder, facilitate early detection of Alzheimer's disease in at-risk but asymptomatic individuals, and reduce the demand for clinical expertise in the diagnosis of Alzheimer's disease, which would improve the accuracy of diagnosis by nonspecialists.
The ideal biological marker would have a high correlation with neuropathological markers of Alzheimer's disease, becoming positive at illness stages that precede the appearance of clinical symptoms. It would reflect the severity of pathological changes in Alzheimer's disease, correlate with the impact of efficacious therapy on its clinical manifestations, and have specificity for the disease with few false positives. The ideal biological marker would also be convenient, with the optimal test being a serological measure, and would have a low cost, allowing wide applicability including screening. Discovery of such a marker is a research priority.
Treatment
The goal of research on Alzheimer's disease is the identification of agents to prevent the occurrence, defer the onset, slow the progression, or improve the symptoms of disease. Progress has been made in this arena, and several agents have been shown to have beneficial effects in the treatment of Alzheimer's disease. Cholinesterase inhibitors improve cognitive symptoms or temporarily reduce the rate of cognitive decline (8,9,10). Vitamin E and selegiline delay the occurrence of important milestones in the course of Alzheimer's disease, including severe impairment, nursing home placement, and death (7). Epidemiologic data suggest that use of estrogen (15) and nonsteroidal anti-inflammatory drugs (16,17) may prevent or delay the onset of the disorder.
These current therapeutic approaches do not address the generation of the beta-amyloid peptide, increasingly regarded as the central pathological event in Alzheimer's disease (18,19). Effective therapy for Alzheimer's disease may involve affecting multiple steps within the putative pathogenic cascade. Important treatment approaches include reducing beta-amyloid generation from the amyloid precursor protein, decreasing beta-amyloid aggregation and formation of beta-pleated sheets, interfering with amyloid-related neurotoxicity, interrupting cell death mechanisms, inhibiting inflammatory response occurring in neuritic plaques, using growth factors, implementing hormonal therapies, and replenishing deficient neurotransmitters. Complete blockade of steps within the amyloid cascade may interfere with normal cerebral metabolic processes, and it is more likely that partial interruptions of multiple steps will have summary or multiplicative effects that have a meaningful impact on the pathogenesis and course of Alzheimer's disease.
Conversion of mild cognitive impairment to Alzheimer's disease is a critical step in the evolution of the disease, and preventing or delaying its occurrence would keep patients at higher levels of functioning for longer periods of time. Identifying individuals who are at high risk for conversion because of memory impairment but who do not have other features of dementia is feasible and could provide the basis for selecting elderly persons most in need of treatment (20,21,22,23). Clinical trials of amyloid-modulating, neuroprotective, and cholinergic compounds are needed to determine their effect on the evolution of this prodromal phase of Alzheimer's disease.
Structured, stage-specific pharmacotherapeutic regimens for symptomatic patients with Alzheimer's disease can be envisioned. The approach to early-stage and midstage patients emphasizes enhancing function and deferring disability through use of combinations of anti-amyloid, anti-inflammatory, cholinergic, and neuroprotective agents. Prolonging the lives of late-stage patients may not be a therapeutic goal of patients and their families, and care of these patients may emphasize comfort care and reduction of behavioral disturbances.
Patients with Alzheimer's disease receive most of their care from family members, and expansion of support services for caregivers is an important aspect of meeting the challenge of Alzheimer's disease.
The neuropsychiatric dimension of Alzheimer's disease also requires increased therapeutic attention. Neuropsychiatric disorders in Alzheimer's disease result in distress to patients and caregivers, premature institutionalization, increased cost of care, and significant compromise of the quality of life of patients and their families (24,25,26,27).
Reported frequencies of neuropsychiatric symptoms among patients with Alzheimer's disease are high: 30 to 50 percent experience delusions, 10 to 25 percent have hallucinations, and 40 to 50 percent have symptoms of depression (28,29). Psychotic patients have greater cognitive impairment, more rapidly progressive dementia, and greater frontal and temporal dysfunction on metabolic imaging (30,31). Psychotic patients also exhibit more agitation, depression, wandering, anger, personality change, family or marital problems, and lack of self-care (32). Even modest reduction in troublesome behaviors can produce substantial improvements in function and quality of life (33), but optimal treatments for delusions, mood changes, agitation, anxiety, and insomnia have not been identified.
Few double-blind, placebo-controlled studies of psychotropic medications in Alzheimer's disease have been accomplished. Treatments developed for patients with primary psychiatric illness and psychiatric symptoms similar to those of Alzheimer's disease have been used to treat patients with Alzheimer's disease. The emergence of novel antipsychotics and new antidepressants requires that these agents be studied specifically among patients with Alzheimer's disease to establish their utility. The observation that cholinergic agents used to enhance cognition in Alzheimer's disease may have beneficial behavioral effects also needs further exploration (34,35,36). Interactions between agents aimed at treating the underlying disease process and those required to treat behavioral disturbances require investigation.
The current research infrastructure for drug discovery in Alzheimer's disease is limited. Biotechnology and pharmaceutical companies are investigating the effects of anti-amyloid and other mechanism-based agents, but human testing of these agents is not yet feasible. Pharmaceutical companies have focused on cholinesterase inhibitors, and preliminary studies of cholinergic receptor agonists, estrogens, and anti-inflammatory agents have been conducted.
The Alzheimer's disease cooperative study funded by the National Institute on Aging is investigating the effects of anti-inflammatory agents on the course of Alzheimer's disease, as well as the utility of melatonin in reducing sleep disturbances and the effectiveness of sodium valproate in treating the agitation associated with Alzheimer's disease. Individual investigators are pursuing studies of a variety of agents with federal funding or support from the Alzheimer's Association or philanthropic organizations.
Discovering more potent therapies depends on expanding the research infrastructure for drug discovery and testing. Transgenic mouse models of Alzheimer's disease will allow more rapid testing of agents, and funds to support expanded transgenic facilities and research are needed. Some therapeutic intervention may be testable in tissue culture or other laboratory preparations.
However, all promising agents will require human testing, and additional funds are needed for this process. Objectives include expanding recruitment of subjects for clinical trials, increasing the number of clinical trials, testing multiple therapeutic agents simultaneously to determine interactive effects, establishing financial incentives for development of drugs that act early in the pathogenic cascade, studying psychotropic agents that reduce behavioral disturbances with few side effects, and investigating the late phases of Alzheimer's disease to determine how best to treat patients with severe dementia syndromes.
Discussion
An integrated continuum of research is necessary to ensure that progress in the laboratory is seamlessly exported to the community and raises the standard of care. This continuum can be envisioned as encompassing four phases: basic science research such as molecular and cellular biology, transitional research that links basic and clinical science and includes studies in animal models of disease and use of nonhuman primates, clinical science involving living human beings or tissue derived from them, and translational science including testing how best to export advances in neuroscience and technology transfer into community practice. This research continuum is illustrated in Table 1, applied to the development of drugs for the disease.
Implementation and support of each step of the continuum are critical if all citizens are to benefit from research discoveries. Advances in basic science lack human value and have no impact on public health if they are not accompanied by advances in clinical and translational science that allow patients to benefit from improved understanding of disease processes and how to intervene in them. Commercial enterprises have taken the lead in promoting advances—in the form of products—to consumers. However, few of the mechanisms developed for technology transfer have been scientifically tested, comparative studies have not been conducted, and consumer purchasing behavior rather than quality of life may be emphasized in these promotional efforts. Raising the level of public health, improving quality of life, and justifying public investment in the scientific enterprise requires a greater investment in clinical and outcomes research accompanying vigorous support of basic research activities.
In summary, the anticipated growth of the number of patients with dementia poses an urgent public health challenge. Improvements in detection, diagnosis, and treatment are needed. There have been marked advances in understanding Alzheimer's disease, and preliminary steps have been taken to develop new therapies. An expanded research commitment is necessary to accelerate drug discovery, and more translational research is needed to ensure that new discoveries are incorporated into clinical practice.
Acknowledgments
This project was supported by grant AG-10123 from the Alzheimer's Disease Center of the National Institute on Aging, the Sidell-Kagan Foundation, a State of California Alzheimer's Disease Research Center grant to the University of California, Los Angeles, and the Department of Veterans Affairs.
Dr. Cummings is professor of neurology, psychiatry, and biobehavioral sciences in the departments of neurology and psychiatry at the University of California, Los Angeles, School of Medicine, 710 Westwood Plaza, Los Angeles, California 90095-1769 (e-mail, [email protected]). Dr. Jeste is professor of psychiatry and neurosciences in the department of psychiatry at the University of California, San Diego, and the Veterans Affairs San Diego Healthcare System. This paper is part of a special section on meeting the mental health needs of the growing population of elderly persons.
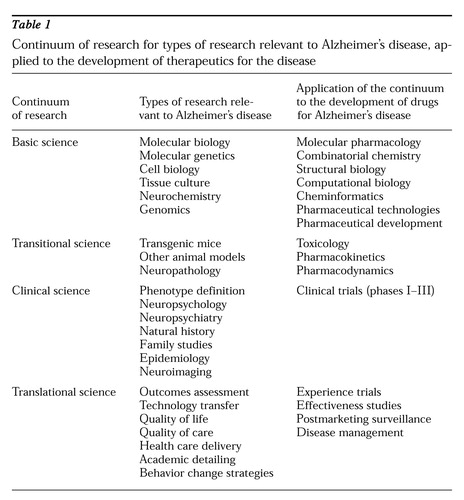 |
Table 1.
Continuum of research for types of research relevant to Alzheimer's disease, applied to the development of therapeutics for the disease
1. Malmgren R: Epidemiology of aging, in The American Psychiatric Press Textbook of Geriatric Neuropsychiatry. Edited by Coffey CE, Cummings JL. Washington, DC, American Psychiatric Press, 1994Google Scholar
2. Evans DA, Funkenstein HH, Albert MS, et al: Prevalence of Alzheimer's disease in a community population of older persons: higher than previously reported. JAMA 262:2551-2556, 1989Crossref, Medline, Google Scholar
3. Jorm AF, Korten AE, Henderson AS: The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatrica Scandinavica 76:465-479, 1987Crossref, Medline, Google Scholar
4. Hill LR, Klauber MR, Salmon DP, et al: Functional status, education, and the diagnosis of dementia in the Shanghai survey. Neurology 43:138-145, 1993Crossref, Medline, Google Scholar
5. Katzman R: Education and the prevalence of dementia and Alzheimer's disease. Neurology 43:13-20, 1993Crossref, Medline, Google Scholar
6. Stern Y, Gurland B, Tatemichi TK, et al: Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 271:1004-1010, 1994Crossref, Medline, Google Scholar
7. Sano M, Ernesto C, Thomas RG, et al: A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. New England Journal of Medicine 336:1216-1222, 1997Crossref, Medline, Google Scholar
8. Cummings JL, Cyrus PA, Bieber F, et al: Metrifonate treatment of the cognitive deficits in Alzheimer's disease. Neurology 50:1214-1221, 1998Crossref, Medline, Google Scholar
9. Farlow M, Gracon SI, Hershey LA, et al: A controlled trial of tacrine in Alzheimer's disease. JAMA 268:2523-2529, 1992Crossref, Medline, Google Scholar
10. Rogers SL, Friedhoff LT, and the Donepezil Study Group: The efficacy and safety of donepezil in patients with Alzheimer's disease: results of a US multicenter, randomized, double-blind, placebo-controlled trial. Dementia 7:293-303, 1996Medline, Google Scholar
11. O'Connor DW, Pollitt PA, Hyde JB, et al: Do general practitioners miss dementia in elderly patients? British Medical Journal 297:1107-1110, 1988Google Scholar
12. Callahan CM, Hendrie HC, Tierney WM: Documentation and evaluation of cognitive impairment in elderly primary care patients. Annals of Internal Medicine 122:422-429, 1995Crossref, Medline, Google Scholar
13. McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939-944, 1984Crossref, Medline, Google Scholar
14. Galasko D, Hansen LA, Katzman R, et al: Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Archives of Neurology 51:888-895, 1994Crossref, Medline, Google Scholar
15. Paganini-Hill A, Henderson VW: Estrogen deficiency and risk of Alzheimer's disease in women. American Journal of Epidemiology 140:256-261, 1994Crossref, Medline, Google Scholar
16. Andersen K, Launer LJ, Ott AA, et al: Do nonsteroidal anti-inflammatory drugs decrease the risk for Alzheimer's disease? The Rotterdam study. Neurology 45:1441-1445, 1995Crossref, Medline, Google Scholar
17. McGeer PL, Schulzer M, McGeer EG: Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology 47:425-432, 1996Crossref, Medline, Google Scholar
18. Cummings JL, Vinters HV, Cole GM, et al: Alzheimer's disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology 51(suppl 1):S2-S17, 1998Google Scholar
19. Hardy JA, Higgins GA: Alzheimer's disease: the amyloid cascade hypothesis. Science 256:184-185, 1992Crossref, Medline, Google Scholar
20. Flicker C, Ferris SH, Reisberg B: Mild cognitive impairment in the elderly: predictors of dementia. Neurology 41:1006-1009, 1991Crossref, Medline, Google Scholar
21. Howieson DB, Dame A, Camicioli R, et al: Cognitive markers preceding Alzheimer's dementia in the healthy oldest old. Journal of the American Geriatrics Society 45:584-589, 1997Crossref, Medline, Google Scholar
22. Linn RT, Wolf PA, Bachman DL, et al: The "preclinical phase" of probable Alzheimer's disease. Archives of Neurology 52:485-490, 1995Crossref, Medline, Google Scholar
23. Teirney MC, Szalai JP, Snow WG, et al: Prediction of probable Alzheimer's disease in memory-impaired patients: a prospective longitudinal study. Neurology 46:661-665, 1996Crossref, Medline, Google Scholar
24. Ferris SH, Steinberg G, Shulman E, et al: Institutionalization of Alzheimer's disease patients: reducing precipitating factors through family counseling. Home Health Care Services Quarterly 8(1):23-51, 1987Google Scholar
25. Finkel SI, Coste e Silva J, Cohen G, et al: Behavioral and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. International Psychogeriatrics 8:497-500, 1996Crossref, Medline, Google Scholar
26. Kaufer DI, Cummings JL, Christine D, et al: Assessing the impact of neuropsychiatric symptoms in Alzheimer's disease: the Neuropsychiatry Inventory Caregiver Distress Scale. Journal of the American Geriatrics Society 46:210-215, 1998Crossref, Medline, Google Scholar
27. Rabins PV, Mace NL, Lucan MJ: The impact of dementia on the family. JAMA 248:333-335, 1982Crossref, Medline, Google Scholar
28. Mega MS, Cummings JL, Fiorello T, et al: The spectrum of behavioral changes in Alzheimer's disease. Neurology 46:130-135, 1996Crossref, Medline, Google Scholar
29. Wragg R, Jeste DV: Overview of depression and psychosis in Alzheimer's disease. American Journal of Psychiatry 146:577-587, 1989Link, Google Scholar
30. Jeste DV, Wragg RE, Salmon DP, et al: Cognitive deficits of patients with Alzheimer's disease with and without delusions. American Journal of Psychiatry 149:184-189, 1992Link, Google Scholar
31. Sultzer DL, Mahler ME, Mandlekern MA, et al: Relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer's disease. Journal of Neuropsychiatry and Clinical Neurosciences 7:476-484, 1995Crossref, Medline, Google Scholar
32. Rockwell E, Jackson E, Vilke G, et al: A study of delusions in a large cohort of Alzheimer disease patients. American Journal of Geriatric Psychiatry 2:157-164, 1994Medline, Google Scholar
33. Shulman E, Steinberg G: Emotional reactions of Alzheimer's caregivers in support group settings. Gerontologist 24:158, 1984Google Scholar
34. Bodick NC, Offen WW, Levey AI, et al: Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Archives of Neurology 54:465-473, 1997Crossref, Medline, Google Scholar
35. Kaufer DI, Cummings JL, Christine D: Effect of tacrine on behavioral symptoms in Alzheimer's disease: an open label study. Journal of Geriatric Psychiatry and Neurology 9:1-6, 1996Crossref, Medline, Google Scholar
36. Raskind MA, Sadowsky CH, Sigmund WR, et al: Effect of tacrine on language, praxis, and noncognitive behavioral problems in Alzheimer disease. Archives of Neurology 54:836-840, 1997Crossref, Medline, Google Scholar



