Regional Differences in Five-Year Mortality After a First Episode of Schizophrenia in Finland
Patients with schizophrenia have an approximately 20% reduced life expectancy compared with the general population ( 1 ). They have an increased risk of death from both natural and unnatural causes ( 2 , 3 ). Among natural causes of death, the most common are cardiovascular and respiratory diseases ( 3 , 4 , 5 ). Suicide is the most common unnatural cause of death ( 3 ), and it usually occurs near the onset of schizophrenia ( 6 , 7 , 8 , 9 ). Excess mortality among schizophrenia patients is explained by patients' unhealthy lifestyle ( 10 ) and by medication side effects that can result in diabetes ( 11 ), obesity ( 12 , 13 , 14 ), and cardiac diseases ( 12 , 15 , 16 , 17 ). Furthermore, untreated or undiagnosed physical illnesses among psychiatric patients may be related to their premature mortality ( 18 , 19 , 20 ). Despite the awareness of these issues, excess mortality among schizophrenia patients has remained an ongoing challenge in health care.
Mortality indicators, particularly the standardized mortality ratio (SMR), are generally used for assessing the outcome, effectiveness, and quality of psychiatric and medical care as well as preventive programs ( 21 , 22 ). SMRs express mortality of patients in relation to the general population, which is usually the entire general population of a country. Although mortality inequities across geographic localities are recognized ( 23 , 24 ), SMRs for psychiatric patients are rarely adjusted for where they live. In a British study, researchers reported increased SMRs in northern regions and decreased SMRs in southern regions of Great Britain ( 25 ). Two Finnish studies found regional differences in mortality within a national schizophrenia population, but SMRs were not calculated ( 26 , 27 ). Recognition of the effect of place of residence on mortality is essential when evaluating the outcome of treatment between regions. Extremely high or low SMRs by region may be of help in identifying factors that may explain the equality and quality of mental health services.
Our register-based study consists of a national sample of patients with first-onset schizophrenia between the years 1995 and 2001 in Finland. The SMR was used as an outcome measure over a five-year follow-up period. SMRs were calculated by gender, age group, and different cause-of-death categories and across 20 hospital districts. Potential years of life lost (PYLLs) were calculated with life expectancy ages as a reference. Special focus was given to suicides and deaths from circulatory diseases, because these are known to be the most common cause-of-death categories in schizophrenia populations.
Methods
Registers
Our study is part of a schizophrenia subproject from the PERFormance, Effectiveness, and Cost of Treatment episodes (PERFECT) project, which is a collaboration between the National Institute for Health and Welfare (THL), five university hospital districts, and the Social Insurance Institution (SII) of Finland ( info.stakes.fi/perfect/EN/index.htm ). Three data sources were used: the national hospital discharge register (FHDR) from THL, the national causes-of-death register from Statistics Finland, and registers of disability pensions and reimbursed medicines from the SII. Linkage of data across registers was performed by using a unique personal identification number. The FHDR contains data on all admissions since 1969 to Finnish inpatient facilities and includes information on treatment, such as dates for hospitalization and diagnoses at discharge ( 28 , 29 , 30 ). The national causes-of-death register contains information from death certificates issued by physicians. The registers from the SII include information on all disability pensions since 1962 and information on patients since 1964 with illnesses eligible for special medication reimbursement. The study protocol was approved by the National Research and Development Centre for Welfare and Health Ethics Committee.
Participants
The study population includes patients whose first episode of schizophrenic illness occurred between January 1, 1995, and December 31, 2001. Three different approaches were used to identify the study participants. First, data for all first-admission patients with schizophrenia ( ICD-9 code 295; ICD-10 codes F20 and F25) as a primary diagnosis were extracted from the FHDR. The onset of schizophrenia was defined as the first admission for schizophrenia (N=3,616). Second, we searched the register for schizophrenia patients with disability pensions, reasoning that some persons with a disability pension from schizophrenia had not been hospitalized because of schizophrenia or psychosis before the pension was granted. If these persons received disability benefits during the study period, they were included in the data (N=622). Third, the data included patients who were admitted to a psychiatric hospital because of psychosis ( ICD-9 codes 297–299; ICD-10 codes F22–F24 and F28–F29) and who later received a diagnosis of schizophrenia as indicated in the FHDR or a disability pension for schizophrenia, according to the SII (N=3,353). The onset of schizophrenia in this case was defined as the first admission date for psychosis. For each eligible patient the follow-up time was five years after the onset of schizophrenia.
Causes of death
The causes-of-death register contains diagnoses according to the ICD-9 (up to 1995) and ICD-10 (from 1996 on) criteria. All deaths are diagnosed by physicians, and a forensic medical examination is called for if the cause of death is uncertain. The Finnish death certification practices have been shown to be reliable for research purposes ( 31 ). In our study, the classification of causes of death was that used by Statistics Finland ( www.tilastokeskus.fi/til/ksyyt/index_en.html ).
In our study, the causes of death were also categorized into natural and unnatural deaths. Unnatural deaths include the codes for suicide ( ICD-9 codes E950-E959B and E959X; ICD-10 codes X60–X84 and Y870), accident ( ICD-9 codes E800–E929 and E970–E990; ICD-10 codes V01ndash;X59, Y10–Y86, Y872, and Y88–Y89), and homicide ( ICD-9 codes E960–969; ICD-10 codes 85–Y09 and Y871). All other codes were defined as natural causes of death.
Hospital districts
In Finland, psychiatric specialist-level care is provided by 21 hospital districts. The Finnish Act on Specialized Medical Care requires that every municipality must belong to one hospital district. The Mental Health Act and Mental Health Decree provide the main guidelines for mental health work ( www.finlex.fi/en/ ). Each municipality must guarantee that persons domiciled in the municipality receive the necessary specialized medical care. Mental health services are primarily organized as noninstitutional care, but there is great variation between municipalities. In our study, hospital district indicates the place of residence of study participants. One hospital district—the Province of Åland—was excluded from this study because its residents could have had hospital admissions abroad.
Statistical analyses
SMRs were calculated with age, gender, place of residence (that is, hospital district), and year of death matched to the general Finnish population as the reference. In addition, a summary measure of premature mortality, PYLL, was calculated, which is the potential number of years of life lost when a person dies prematurely from any cause. The data are presented as a standardized rate per 100,000 population, with life expectancy ages as a reference for both males and females (Statistics Finland). The denominator is the respective age- and sex-matched population at risk of premature death. At the hospital district level, Pearson correlation was used to examine the association of SMRs with general morbidity indices ( raportit.kela.fi/ibi_apps/WFServlet?IBIF_ex=WIT079AL ), with population per psychiatric hospital beds and realized working years of the physicians (meaning the number of years spent working in psychiatric specialized care; data provided by the Association of Finnish Local and Regional authorities), as well as with degree of urbanization and socioeconomic status, as indicated by unemployment rate, income tax, and social assistance per citizen (Statistics Finland). Numbers of outpatient visits in specialized care and in the municipal mental health sector were obtained from the SOTKAnet indicator bank by THL ( uusi.sotkanet.fi/portal/page/portal/etusivu ). Statistical analyses were performed with SAS 9.1 and SPSS 15.0 for Windows packages.
Results
The mean±SD age of the total sample of 7,591 patients was 33.5±12.6; mean age was 31.94±11.82 for males and 35.77±13.23 for females (range seven to 65 years). Males (N=4,403) constituted 58% of the sample. A total of 403 (5%) patients who had experienced a first episode of schizophrenia died during the five-year follow-up: 286 (6%) males and 117 (4%) females. Of all deaths, 191 (47%) were natural deaths: 131 (46%) for males and 60 (51%) for females.
SMRs by causes of death and gender
Patients who had experienced a first episode of schizophrenia had a significantly increased all-cause mortality and mortality from natural and unnatural causes compared with the general population ( Table 1 ). The most common natural cause of death was circulatory diseases (85 of 191 patients, 44%), distributed as follows: ischemic heart disease ( ICD-10 codes I20–I25), 43 patients (51%); other forms of heart disease (codes I30–I52), 20 patients (24%); cerebrovascular diseases (codes I60–I69), 11 patients (13%); pulmonary heart diseases and diseases of pulmonary circulation (codes I26–I28), five patients (6%); diseases of veins, lymphatic vessels, and lymph nodes (codes I80–I89), four patients (5%); and hypertensive heart diseases (codes I10–I15), two patients (2%). The highest SMR (24.8) for natural cause of death was found with ill-defined and unknown causes.
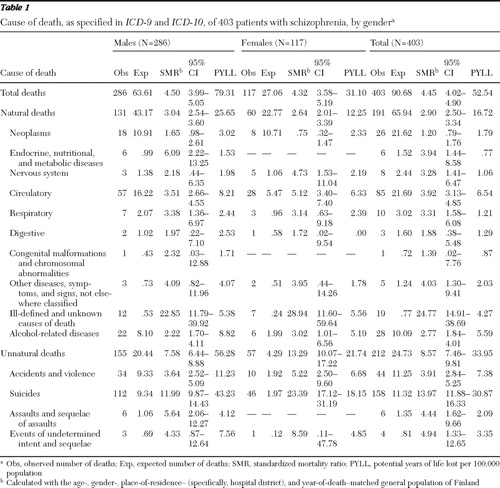 |
Of all unnatural causes of death, suicide was the most common (158 of 212 patients, 75%), and it had the highest SMR (14.0). Similarly, both for males and females the most common natural cause of death was circulatory diseases (57 males, 44%; 28 females, 47%), and the highest SMR (males, 22.85; females, 28.94) was found with ill-defined and unknown causes of death. Furthermore, suicide was the most common category for both males and females (112 males, 72%; 46 females, 81%) and it had the highest SMR (males, 12.0; females, 23.4) among the unnatural causes of death. The total PYLL was 79.31 for males, 31.10 for females, and 52.54 for the total group, and the highest PYLL per 100,000 population was found for suicides, at 30.87.
Mortality by age group and gender
Figure 1 presents the age-specific SMRs for all causes, circulatory diseases, and suicides in the total data set and by gender. All-cause mortality was significantly increased compared with the general population over the five-year follow-up period in all age groups for the total data and for males. All-cause SMRs were significantly higher for females, except those aged 15–19, 35–39, and 65–69. The highest SMRs were found in age group 25–29 for the total group and among male patients, and in age group 20–24 among females. In the total group and for males and females as separate groups, SMRs decreased with increasing age.

Mortality for suicides over the five-year follow-up period was significantly increased compared with the general population in all age groups in the total data set and for both genders, except males aged 55–59 and 65–69 and females aged 15–19. The highest SMRs were found in age groups 15–19 and 65–69 in the total data set. The SMR among males was highest at ages 15–19, and the SMR among females was highest in age group 65–69.
In the total data set, mortality for circulatory diseases was significantly increased compared with the general population in all age groups over the five-year follow-up period, with the exception of age groups 35–39 and 65–69 years, and the highest SMR was seen among 20- to 24-year-olds. Among males, SMRs increased between ages 20 and 29 and between ages 45 and 54 and were highest among 25- to 29-year-olds. Among females, SMRs were highest between ages 20 and 39 and between ages 50 and 64, being highest at ages 20–24 years.
SMRs by hospital district
All-cause mortality of schizophrenia patients was significantly increased compared with the general population in 18 of 20 (90%) hospital districts. [Maps showing SMRs by region are available as an online supplement to this article at ps.psychiatryonline.org .] Statistically nonsignificant SMRs were observed in northern Finland and northwestern Finland. In all other hospital districts SMRs varied from 2.9 to 7.2 (SMR= 4.45 for the whole country). The highest SMR for schizophrenia patients was observed in southwestern Finland.
The suicide mortality of schizophrenia patients was significantly increased in 16 of 20 (80%) hospital districts. In two hospital districts there were no suicides. Nonsignificant SMRs for suicide were seen in northern Finland and in one region in southeastern Finland. In all other hospital districts SMRs for suicide varied from 7.8 to 22.9 (SMR=14.0 for the whole country). The highest SMRs were observed in western Finland, southwestern Finland, and eastern Finland.
Increased SMRs for schizophrenia patients with circulatory diseases were observed in ten out of 20 (50%) hospital districts. One hospital district did not have any cardiac deaths. Nonsignificant SMRs were observed in southern and western regions of Finland. SMRs for hospital districts varied from 1.3 to 8.8 (SMR=3.9 for the whole country). The highest SMRs were observed in southeastern Finland and areas in southwestern Finland. The SMRs for circulatory system diseases were not correlated with the morbidity index for coronary artery diseases in the general population.
Analyses at the hospital district level showed that various SMRs were correlated with indicators for morbidity, psychiatric services, socioeconomic status, and degree of urbanization ( Table 2 ). The only statistically significant correlation was found between the overall SMRs and psychiatrists' realized working years. When all indicators were entered simultaneously in a multivariate regression model, none of them explained the variation in the all-cause SMRs and the SMRs for suicide. SMRs for circulatory system diseases were, however, predicted by unemployment rate in the hospital district ( β =1.36, t=2.29, df=19, p=.043).
 |
Discussion
In our five-year follow-up study, excess mortality was clearly observed for patients admitted for the first time with schizophrenia. All-cause mortality SMRs decreased with older age, approaching the mortality of the general population. The most common unnatural cause of death was suicide, whereas ill-defined and unknown causes were most common among natural causes of death. Variation in mortality between hospital districts was evident.
The mortality for natural causes was almost threefold higher than that of the general population, which is higher than in earlier studies ( 3 , 4 , 5 ). The result may indicate a rising trend in the mortality gap between patients with schizophrenia and the general population, a finding that was also indicated in the meta-analysis conducted by Saha and colleagues ( 3 ). Methodological differences complicate comparison of our findings with findings from previous studies. In earlier studies, the onset of schizophrenia was defined as starting from first hospitalization for schizophrenia ( 22 , 32 , 33 , 34 ). Furthermore, previous studies used a mixed population of patients who had experienced only their first episode of illness and those whose illness was chronic ( 2 , 21 , 35 ) or included only patients with chronic schizophrenia ( 36 , 37 ). Our study's first-onset patients were younger than the other cohort, which would by itself increase the SMRs. The excess mortality in schizophrenia is worse in the beginning of the disease.
In our data, the most common cause of death was circulatory diseases, the mortality being fourfold higher than that of the general population, which is higher than in previous studies ( 3 , 4 , 34 , 38 ). Patients with schizophrenia have many lifestyle risk factors for cardiovascular diseases ( 39 ). Medication side effects could cause metabolic disorders ( 14 , 40 , 41 ) and sudden cardiac death ( 15 , 42 ). Metabolic disorders are often untreated among patients with schizophrenia ( 43 ), and the quality of treatment of cardiovascular diseases among them may be poorer than in the general population ( 18 , 20 ). About one-third of the patients have depressive symptoms ( 44 ) and most have cognitive deficits ( 45 ), both of which decrease their level of function and may decrease their motivation and capacity to take care of themselves.
An interesting finding was that the mortality for ill-defined and unknown causes was almost 25-fold higher than that of the general population, compared with 14-fold higher in an earlier study ( 46 ). In Finland, only 1%–2% of causes of death remain unknown ( 47 ). A Swedish population-based study reported an increased proportion of patients with schizophrenia whose bodies were not discovered until many days after death ( 48 ). This indicates a high level of social isolation and unavailability of adequate mental health care. It is also possible that some of these deaths are misclassified as suicides.
The SMRs for unnatural causes was nearly ninefold, which is in agreement with previous research ( 3 ), although fourfold SMRs have also been reported ( 4 , 5 ). In our study the SMR for suicides was as high as 14-fold compared with that of the general population. Suicide mortality is known to increase in younger age groups among persons with schizophrenia ( 22 ). Our high SMRs for unnatural causes and suicides may also be due to the sampling of patients with first-onset schizophrenia. The highest risk for suicide among first-onset schizophrenia patients is known to occur within one year after admission ( 8 , 22 , 32 , 49 ). The highest PYLLs were found with suicides, because of the predominance of young schizophrenia patients in this death category ( 50 ).
In our data set, the SMRs for all causes, suicides, and circulatory diseases tended to be higher compared with the general population among females than males in almost all age groups. In a Swedish study, SMRs for natural causes for women in all age groups were also higher compared with those for men ( 22 ). Furthermore, higher SMRs for women by age group were found in a study by Mortensen and Juel ( 32 ). In their study, however, there were no cardiovascular deaths among women under the age of 50, whereas in our study SMRs for circulatory diseases were especially high among 20- to 44-year-old women. Conversely, compared with a control group from the general population, men with schizophrenia have been shown to have a higher risk of coronary heart diseases than women ( 34 , 51 ). Our findings may indicate that the health gap between schizophrenia patients and the general population is wider among women than men. Female schizophrenia patients may not have benefited from the improvements in health outcomes that have been available for the general population.
The mean age of 34 years for a sample of patients with first-onset schizophrenia is quite high. Our study sample differed from previous studies of patients with first-onset schizophrenic illness because it included not only patients with their first hospital admission for schizophrenia but also those with schizophrenia who were receiving disability benefits. All patients with disability benefits had been granted early retirement because of schizophrenia before their first hospital admission for the illness. In our study the onset age among patients with disability benefits was higher (40.9±10.58) than the age of first-admission patients (32.9±12.53), which explains why age of onset was higher in our study than is reported in earlier studies ( 52 , 53 , 54 ). On the other hand, the age of onset in our study sample agrees with the previous finding that the time lag between the occurrence of the first sign of a schizophrenia and first hospital admission with a schizophrenia diagnosis averages 6.3 years ( 55 ).
This study showed variations in SMRs between hospital districts in Finland. Lack of association between SMRs and morbidity indices, however, indicated that the general health status of the population in a hospital district does not explain the mortality. No psychiatric service, socioeconomic indicator, or degree of urbanization was related to all-cause SMR or to suicide SMR. Unemployment rate in the hospital district was, however, associated with an increased SMR for circulatory system diseases, which is in line with previous studies ( 56 , 57 , 58 ). In general, our findings suggest that regional variations in SMRs are not associated with population characteristics or psychiatric health care resources of a hospital district but rather with the content and quality of psychiatric treatment. In Finland, hospital districts have to organize medical care for those living within the district, and all citizens have the same legal right to receive the medical care that is necessary. Patients should thus get equally good service and treatment in every hospital district in Finland. In reality, municipalities' and entire hospital districts' outpatient services, resources, and treatment vary between regions, which may at least partly explain the regional variations in SMRs in our study.
Strengths of the study
This study has several strengths. First, Finnish national registers have been shown to be valid tools for scientific research ( 29 , 30 , 59 ). The national registers cover the whole country, and a unique identification number assigned to every Finnish citizen ensures the full coverage and quality of data linkages. It is thus possible to produce useful information on health system performance. Second, SMRs were calculated with the age-, gender-, and region-matched general Finnish population as a reference. Our study provided regionally adjusted SMRs, which is a novel approach and may have produced more accurate estimates of SMRs than those reported in previous studies. Third, our sampling technique is a strength of this study. Our definition of onset justifies assuming that patients with schizophrenia were in the same stage of disease when the follow-up period began. Fourth, first-episode cohort studies provide the most accurate estimate of the excess mortality of schizophrenia because other cohorts include participants who have already survived the period of greatest excess mortality ( 4 ).
Limitations of the study
A limitation of this study is that Finnish health and social welfare registers were originally collected for administrative purposes ( 60 ). Register-based data do not include details about treatment, life events before death, patients' lifestyle, and how their outpatient treatment was arranged. Another limitation is that comparison between hospital districts is difficult because districts contain several psychiatric hospitals and municipalities with different types and amounts of health care resources. Also, our findings have some risk of type I error because of the many statistical tests performed and risk of type II error because of the small samples in some subgroups. Finally, the number of first-onset schizophrenia patients without any lifetime history of hospitalization or disability pension remains unknown, because national registers do not cover treatment in outpatient settings. Consequently, there might be some patients who had outpatient contacts before inpatient treatment who were not accounted for.
Conclusions
In general, excess mortality in first-onset schizophrenia was observed in the first five years of illness in all age groups, especially in the case of suicides and cardiovascular deaths. These findings were established after taking into account the general health status of persons living in the same region as the patients. In clinical work attention should be paid to suicide risk and the somatic, especially cardiovascular, status of schizophrenia patients. Regional differences in mortality seem to exist, but further research on causal factors and pathways in early deaths is needed.
Acknowledgments and disclosures
This study was supported in part by a research grant from the Academy of Finland and a grant from Oy H. Lundbeck Ab. The authors thank Mikko Peltola, M.Sc., for register linkage and preparation of the data set.
1. Hennekens CH, Hennekens AR, Hollar D, et al: Schizophrenia and increased risks of cardiovascular disease. American Heart Journal 150:1115–1121, 2005Google Scholar
2. Brown S, Inskip H, Barraclough B: Causes of the excess mortality of schizophrenia. British Journal of Psychiatry 177:212–217, 2000Google Scholar
3. Saha S, Chant D, McGrath J: A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of General Psychiatry 64:1123–1131, 2007Google Scholar
4. Brown S: Excess mortality of schizophrenia: a meta-analysis. British Journal of Psychiatry 171:502–508, 1997Google Scholar
5. Harris EC, Barraclough B: Excess mortality of mental disorder. British Journal of Psychiatry 173:11–53, 1998Google Scholar
6. Palmer BA, Pankratz VS, Bostwick JM: The lifetime risk of suicide in schizophrenia: a reexamination. Archives of General Psychiatry 62:247–253, 2005Google Scholar
7. Qin P, Nordentoft M: Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Archives of General Psychiatry 62:427–432, 2005Google Scholar
8. Nordentoft M, Laursen TM, Agerbo E, et al: Change in suicide rates for patients with schizophrenia in Denmark, 1981–97: nested case-control study. British Medical Journal 329:261, 2004Google Scholar
9. Bertelsen M, Jeppesen P, Petersen L, et al: Suicidal behaviour and mortality in first-episode psychosis: the OPUS trial. British Journal of Psychiatry Supplement 51:s140–s146, 2007Google Scholar
10. Goff DC, Cather C, Evins AE, et al: Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. Journal of Clinical Psychiatry 66:183–194, 2005Google Scholar
11. Sernyak MJ, Leslie DL, Alarcon RD, et al: Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. American Journal of Psychiatry 159:561–566, 2002Google Scholar
12. Koponen H, Saari K, Savolainen M, et al: Weight gain and glucose and lipid metabolism disturbances during antipsychotic medication: a review. European Archives of Psychiatry and Clinical Neuroscience 252:294–298, 2002Google Scholar
13. Volavka J, Czobor P, Sheitman B, et al: Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. American Journal of Psychiatry 159:255–262, 2002Google Scholar
14. Green AI, Patel JK, Goisman RM, et al: Weight gain from novel antipsychotic drugs: need for action. General Hospital Psychiatry 22:224–235, 2000Google Scholar
15. Koponen H, Alaräisänen A, Saari K, et al: Schizophrenia and sudden cardiac death: a review. Nordic Journal of Psychiatry 62:342–345, 2008Google Scholar
16. Hennessy S, Bilker WB, Knauss JS, et al: Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. British Medical Journal 325:1070, 2002Google Scholar
17. Daumit GL, Goff DC, Meyer JM, et al: Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophrenia Research 105:175–187, 2008Google Scholar
18. Druss BG, Bradford DW, Rosenheck RA, et al: Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA 283:506–511, 2000Google Scholar
19. Druss BG, Bradford WD, Rosenheck RA, et al: Quality of medical care and excess mortality in older patients with mental disorders. Archives of General Psychiatry 58:565–572, 2001Google Scholar
20. Lawrence DM, Holman CD, Jablensky AV, et al: Death rate from ischaemic heart disease in western Australian psychiatric patients 1980–1998. British Journal of Psychiatry 182:31–36, 2003Google Scholar
21. Ringbäck Weitoft G, Gullberg A, Rosen M: Avoidable mortality among psychiatric patients. Social Psychiatry and Psychiatric Epidemiology 33:430–437, 1998Google Scholar
22. Ösby U, Correia N, Brandt L, et al: Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophrenia Research 45:21–28, 2000Google Scholar
23. Papastergiou P, Rachiotis G, Polyzou K, et al: Regional differences in mortality in Greece (1984–2004): the case of Thrace. BMC Public Health 8:297, 2008Google Scholar
24. Saarela J, Finnäs F: Cause-specific mortality at young ages: lessons from Finland. Health Place 14:265–274, 2008Google Scholar
25. Lewis G, Booth M: Regional differences in mental health in Great Britain. Journal of Epidemiology and Community Health 46:608–611, 1992Google Scholar
26. Karvonen M, Peltola M, Isohanni M, et al: PERFECT—schitzophrenia: PERFormance, Effectiveness, and Cost of Treatment [in Finnish]. Helsinki, Finland, Stakes, 2008Google Scholar
27. Salokangas RK, Helminen M, Koivisto A, et al: Mortality of patients with schizophrenia in health care districts [in Finnish]. Suomen Lääkärilehti 63:3759–3766, 2008Google Scholar
28. Pihlajamaa J, Suvisaari J, Henriksson M, et al: The validity of schizophrenia diagnosis in the Finnish hospital discharge register: findings from a 10-year birth cohort sample. Nordic Journal of Psychiatry 62:198–203, 2008Google Scholar
29. Moilanen K, Veijola J, Läksy K, et al: Reasons for the diagnostic discordance between clinicians and researchers in schizophrenia in the northern Finland 1966 birth cohort. Social Psychiatry and Psychiatric Epidemiology 38:305–310, 2003Google Scholar
30. Isohanni M, Mäkikyrö T, Moring J, et al: A comparison of clinical and research DSM-III-R diagnoses of schizophrenia in a Finnish national birth cohort: clinical and research diagnoses of schizophrenia. Social Psychiatry and Psychiatric Epidemiology 32:303–308, 1997Google Scholar
31. Lahti RA, Penttilä A: The validity of death certificates: routine validation of death certification and its effects on mortality statistics. Forensic Science International 115:15–32, 2001Google Scholar
32. Mortensen PB, Juel K: Mortality and causes of death in first admitted schizophrenic patients. British Journal of Psychiatry 163:183–189, 1993Google Scholar
33. Heilä H, Haukka J, Suvisaari J, et al: Mortality among patients with schizophrenia and reduced psychiatric hospital care. Psychological Medicine 35:725–732, 2005Google Scholar
34. Laursen TM, Munk-Olsen T, Nordentoft M, et al: Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. Journal of Clinical Psychiatry 68:899–907, 2007Google Scholar
35. Black DW: Mortality in schizophrenia—the Iowa record-linkage study: a comparison with general population mortality. Psychosomatics 29:55–60, 1988Google Scholar
36. Salokangas RK, Honkonen T, Stengard E, et al: Mortality in chronic schizophrenia during decreasing number of psychiatric beds in Finland. Schizophrenia Research 54:265–275, 2002Google Scholar
37. Räsänen S, Hakko H, Viilo K, et al: Excess mortality among long-stay psychiatric inpatients in northern Finland: a challenge for health care. Duodecim 120:179–180, 2004Google Scholar
38. Joukamaa M, Heliovaara M, Knekt P, et al: Mental disorders and cause-specific mortality. British Journal of Psychiatry 179:498–502, 2001Google Scholar
39. Brown S, Birtwistle J, Roe L, et al: The unhealthy lifestyle of people with schizophrenia. Psychological Medicine 29:697–701, 1999Google Scholar
40. Lund BC, Perry PJ, Brooks JM, et al: Clozapine use in patients with schizophrenia and the risk of diabetes, hyperlipidemia, and hypertension: a claims-based approach. Archives of General Psychiatry 58:1172–1176, 2001Google Scholar
41. Reist C, Mintz J, Albers LJ, et al: Second-generation antipsychotic exposure and metabolic-related disorders in patients with schizophrenia: an observational pharmacoepidemiology study from 1988 to 2002. Journal of Clinical Psychopharmacology 27:46–51, 2007Google Scholar
42. Ray WA, Meredith S, Thapa PB, et al: Antipsychotics and the risk of sudden cardiac death. Archives of General Psychiatry 58:1161–1167, 2001Google Scholar
43. Nasrallah HA, Meyer JM, Goff DC, et al: Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophrenia Research 86:15–22, 2006Google Scholar
44. Conley RR, Ascher-Svanum H, Zhu B, et al: The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophrenia Research 90:186–197, 2007Google Scholar
45. Murray GK, Jones PB, Moilanen K, et al: Infant motor development and adult cognitive functions in schizophrenia. Schizophrenia Research 81:65–74, 2006Google Scholar
46. Allebeck P, Wistedt B: Mortality in schizophrenia: a ten-year follow-up based on the Stockholm County Inpatient Register. Archives of General Psychiatry 43:650–653, 1986Google Scholar
47. Jääskeläinen A: Autopsy in forensic medicine and the protection of law. Duodecim 119:1265–1272, 2003Google Scholar
48. Nilsson LL, Logdberg B: Dead and forgotten: postmortem time before discovery as indicator of social isolation and inadequate mental healthcare in schizophrenia. Schizophrenia Research 102:337–339, 2008Google Scholar
49. Limosin F, Loze JY, Philippe A, et al: Ten-year prospective follow-up study of the mortality by suicide in schizophrenic patients. Schizophrenia Research 94:23–28, 2007Google Scholar
50. Marlow AK: Potential years of life lost: what is the denominator? Journal of Epidemiology and Community Health 49:320–322, 1995Google Scholar
51. Goff DC, Sullivan LM, McEvoy JP, et al: A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophrenia Research 80:45–53, 2005Google Scholar
52. Bottlender R, Strauss A, Moller HJ: Impact of duration of symptoms prior to first hospitalization on acute outcome in 998 schizophrenic patients. Schizophrenia Research 44:145–150, 2000Google Scholar
53. Bottlender R, Sato T, Jager M, et al: The impact of the duration of untreated psychosis prior to first psychiatric admission on the 15-year outcome in schizophrenia. Schizophrenia Research 62:37–44, 2003Google Scholar
54. Fennig S, Rabinowitz J, Fennig S: Involuntary first admission of patients with schizophrenia as a predictor of future admissions. Psychiatric Services 50:1049–1052, 1999Google Scholar
55. Maurer K, Hafner H: Methodological aspects of onset assessment in schizophrenia. Schizophrenia Research 15:265–276, 1995Google Scholar
56. Harris DE, Aboueissa AM, Hartley D: Myocardial infarction and heart failure hospitalization rates in Maine, USA: variability along the urban-rural continuum. Rural and Remote Health 8:980, 2008Google Scholar
57. Lynch JW, Kaplan GA, Cohen RD, et al: Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality, and acute myocardial infarction? American Journal of Epidemiology 144:934–942, 1996Google Scholar
58. Petrelli A, Gnavi R, Marinacci C, et al: Socioeconomic inequalities in coronary heart disease in Italy: a multilevel population-based study. Social Science and Medicine 63:446–456, 2006Google Scholar
59. Poikolainen K: Accuracy of hospital discharge data: five alcohol-related diseases. Drug and Alcohol Dependence 12:315–322, 1983Google Scholar
60. Gissler M, Haukka J: Finnish health and social welfare registers in epidemiological research [in Finnish]. Norsk Epidemiologi 14:113–120, 2004Google Scholar



