Effectiveness of Telephone-Based Referral Care Management, a Brief Intervention to Improve Psychiatric Treatment Engagement
Mental disorders or substance use problems are compounded not only by the complexity in tailoring effective psychiatric treatments to the individual but also by the difficulty in initiating the patient's engagement in treatment ( 1 ). Accordingly, even the most effective clinical psychiatric treatment becomes unattainable when patients do not engage in care. Prior work suggests that approximately half of patients receiving psychiatric appointments adhere to their initial appointment ( 2 ). Moreover, across the United States, approximately 48% to 62% of individuals with depression problems receive recommended care, although only 5% to 48% of individuals with substance abuse problems do ( 3 , 4 , 5 , 6 , 7 ). Outcomes of treatment nonattendance imply not only a high rate of patients who are not receiving necessary psychiatric care but also superfluous financial health care costs and inefficient use of psychiatric staffing ( 1 ).
Research to identify subgroups of patients who are at risk of treatment nonattendance has been inconclusive. Among veterans, research has failed to identify individual demographic characteristics, psychiatric care history, and presence and severity of psychiatric conditions as predictive factors of psychiatric treatment adherence ( 2 ). Although not shown in this earlier study, there has been some indication that patients with co-occurring psychiatric and substance problems are least likely to adhere to treatment ( 8 ). These inconclusive results could imply a need to use more patient-centered strategies to increase psychiatric treatment engagement, rather than group identifiers to target patients least likely to engage in care.
Thus an intervention modeled after brief motivational interviewing (BMI) ( 9 ) that strategically focuses on individual patient experiences and attitudes was developed to increase engagement in psychiatric treatment. As defined by Rollnick and Miller ( 9 ), "motivational interviewing is a directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence," and brief intervention is an abbreviated version of motivational interviewing for eased implementation in medical settings. The key components of BMI modeled for this study were the concise and brief presentation of the problem and the discussion and resolution of the ambivalent nature of behavioral change, with the overall direction guided by the patient.
The intervention implemented in this study—telephone-based referral care management (TBR-CM)—was developed as a supplementary clinical service to the Behavioral Health Laboratory (BHL) (www.va.gov/visn4mirecc/bhl) ( 2 ) at the Philadelphia Veterans Affairs Medical Center (PVAMC). The BHL is a clinical service that assists primary care clinicians by bridging the gap between primary and psychiatric services by evaluating and managing patient psychiatric symptoms. TBR-CM utilizes telephone-based BMI sessions to discuss psychiatric symptoms, psychiatric consequences, treatment benefits, and treatment attendance planning to improve motivation and reduce perceived barriers to treatment attendance. Research evidence from the telephone-based disease management trial ( 10 ) and from the "translating initiatives for depression into effective solutions" trial ( 11 ) indicate that telephone-based disease management modules can effectively manage psychiatric symptoms of patients with less complicated illnesses. Further, research literature indicates that simple reminders by mail and phone call ( 1 ) or assistance with appointment scheduling ( 12 ), without motivational components, do not effectively improve psychiatric treatment rates. As a result, this study was designed to expand the treatment areas of disease management models into management of referral care beyond a simple reminder system. This is among the first studies to examine the extension of the disease management model for the management of psychiatric referral care.
To test the effectiveness of TBR-CM, we conducted a randomized controlled trial in a sample of adult male veterans with depression or substance abuse or both, which warranted specialty psychiatric treatment. We hypothesized that participants randomly assigned to the intervention group for receiving TBR-CM would have higher rates of treatment attendance. Intervention effectiveness was also examined to determine whether treatment attendance outcomes varied among age and diagnostic groups.
Methods
This study was conducted within the Mental Illness Research, Education and Clinical Center at the PVAMC in collaboration with associated primary care clinicians. Best-practice recommendations require that PVAMC patients be screened annually for selected psychiatric symptoms. PVAMC primary care clinicians can refer patients who screen positive for psychiatric symptoms, such as depression and substance abuse, to the BHL for further evaluation. BHL psychiatric status evaluations are then used as a tool for psychiatric treatment management planning for the patient.
From September 2005 through May 2006, all veteran patients who completed a BHL evaluation were eligible for study participation. Inclusion and exclusion study criteria were designed to capture a representative sample of patients in need of psychiatric treatment that would not interfere with any preexisting treatment. Participants were required to be 18 years or older; to demonstrate severe depressive disorder (as indicated by a score of 20 or higher on the Patient Health Questionnaire), alcohol abuse or dependence, or self-reported regular illicit drug use in the past year (using drugs more than ten times, excluding marijuana), as determined by the BHL; to accept a referral to specialty psychiatric care; and to have been inactive in psychiatric treatment within the past 12 months. Patients were excluded from participation if they did not speak fluent English or if they had severe cognitive impairment, as determined by a Brief Memory Test score of 16 or higher.
Consent
After the BHL evaluation, eligible participants were given a complete oral description of the study and asked to orally consent to participating. The PVAMC Institutional Review Board approved the use of oral consent because of time constraints within the study design (requiring written consent would have skewed the results to the patients who could attend a consent session and thus would have reduced generalizability) and minimal risk associated with study participation.
Randomization
Participants (projected N=120) were randomly assigned in equal numbers to either usual care (control) or intervention care (TBR-CM) via computerized assignment, developed though SAS 9.1 and implemented by research assistants. This design allows sufficient power (.80) to detect a medium (.25) to large (.40) effect size at α =.05 ( 13 ) between the intervention group and the control group. Randomization was stratified on the basis of three diagnostic groups: severe depression, substance abuse, or comorbid conditions (severe depression and substance abuse). Stratification by diagnostic groups was implemented to ensure a balanced representation of diagnostic groups across randomization; target enrollment for each diagnostic group was 40 participants.
Usual care procedures
Participants assigned to usual care received routine clinical care. After a psychiatric appointment was scheduled, the BHL summary report was sent to the patient's primary care clinician, a letter was mailed to the patient's home with upcoming appointment information, and an automated call, not by a live person, was placed two to three days before the appointment that stated the address, date, and time of the appointment. Participants in usual care were not systematically contacted regarding missed appointments.
TBR-CM intervention procedures
Participants assigned to intervention care received the same initial care as those in usual care, with the addition of TBR-CM. As part of the intervention, participants received one or two phone intervention sessions, each lasting on average 15 minutes, with a behavioral health specialist. The behavioral health specialist was part of a group of registered nurses with several years of experience conducting BMI and disease management. The specialist was supervised weekly and evaluated on a regular basis through regular meetings and audio-evaluations by a BMI-trained psychiatrist.
TBR-CM was designed to assess a holistic depiction of each participant's treatment goals and symptom verification. The discussion then focused on the participant's attitudes toward personal consequences of drinking or depression, positive consequences of reducing and controlling depression and substance use, and positive and negative consequences of participating in psychiatric treatment. Individual barriers to treatment were also assessed to facilitate proactive problem solving to aid participants in overcoming factors that may impede their treatment attendance. The final component of care is an agreement describing the participant's intent to attend his or her psychiatric appointment. The entire intervention is laid out in a workbook form (manualized and piloted) to guide the session, but it also allows the behavioral health specialist to note individual information discussed during the phone session. After the session, the completed workbook, including the nurse's notation of whether the patient made an oral agreement, was mailed to the patient with a cover letter containing information about the upcoming psychiatric appointment.
Intervention participants who attended their first psychiatric appointment received a closure letter reinforcing their treatment attendance with motivational components aimed at attending future appointments. For those who did not attend their first psychiatric appointment, a new appointment was scheduled and the intervention was reimplemented. The second iteration focused on overcoming barriers that contributed to earlier appointment nonadherence. This follow-up session also was accompanied by a supporting workbook and notation about the patient's agreement, mailed to each participant. Regardless of the attendance status for the second appointment, a closure letter supporting treatment participation was mailed to each participant, with no further contact by the behavioral health specialist. Participants who were unable to be contacted for the intervention were sent a letter informing them that a behavioral health specialist had attempted to make contact and another letter reminding them of upcoming scheduled psychiatric appointments.
Research assessments
The measures included in the BHL evaluation, serving as the baseline interview, were the Patient Health Questionnaire (version 9) for depression ( 14 ); Mini-International Neuropsychiatric Interview ( 15 ) modules for mania, psychosis, panic disorder, generalized anxiety disorder, posttraumatic stress disorder (PTSD), and alcohol abuse and dependence; list of current antidepressant medications; measure of alcohol use using a seven-day timeline follow-back ( 16 ); past and current use of illicit substances; five-item Paykel scale for 12-month suicide ideation ( 17 ); history of depression episodes; Medical Outcomes Study 12-item short form ( 18 ) for mental health and physical health functioning; and Blessed Memory Test ( 19 ) to assess severe cognitive deficits. The baseline interview takes approximately 30 minutes to complete. For detailed descriptions of BHL procedures, see Oslin and colleagues ( 2 ) or the BHL operations manual (available on request from the corresponding author).
Psychiatric appointment attendance was monitored via electronic medical records for all participants, who were included in the PVAMC centralized computer medical record system. This database reflects whether the appointment was attended and provides explanation for missed appointments (for example, patient no-show or the clinic rescheduled the appointment). Trained research assistants accessed this database and recorded psychiatric appointment attendance for six months from the date of the patient's consent to participate in the study. In addition, the behavioral health specialist maintained a tracking system containing frequency of contact for intervention participants only.
Data analysis
Chi square tests and analyses of variance were analyzed in SAS 9.1. Random assignment served as the primary independent variable. Treatment adherence was the primary dependent variable, measured as attendance at the scheduled psychiatric appointment and total number of appointments kept over six months. Attendance was monitored for all study participants by use of computerized follow-up. Treatment adherence effects were examined for interaction effects for each diagnostic group (depression versus substance use problems versus co-occurring substance use problems and psychiatric disorder) and age group (younger, that is, ≤54 years, versus older, that is, ≥55 years) in combination with randomization assignment and, for intervention participants only, frequency of behavioral health specialist contacts.
Results
Sample characteristics
A total of 169 patients who completed the BHL evaluation were identified as needing a referral to specialty psychiatric care. From this total number of eligible participants, 42 (25%) refused study participation and 14 (8%) refused a psychiatric appointment. With the exception of nonrandomized patients' being less likely to smoke (69, or 60%, versus 24, or 43%) ( χ2 =5, df=1, p<.03), there were no other demographic or psychiatric diagnostic differences between randomly assigned and nonparticipating eligible patients. The remaining 113 (67%) constituted the study sample and were randomly assigned. The sample was seven fewer than target enrollment because of less representation of patients with co-occurring disorders during the study period. The study sample demographically ( Table 1 ) represents mostly black (N=71, 63%), male, non-Hispanic adults with a wide age range (22–83 years). Most were married or partnered, not living alone, and smokers, and for the most part not involved in paid employment or perceived to be making sufficient income, although they reported a great deal of perceived support. Clinically, the mental and physical functioning of the sample was below standard averages. Most patients reported that they consumed alcohol, used illicit drugs, and experienced anxiety and PTSD symptoms. Across randomization groups (57 intervention patients and 56 usual care patients), there were no significant differences in characteristics ( Table 1 ).
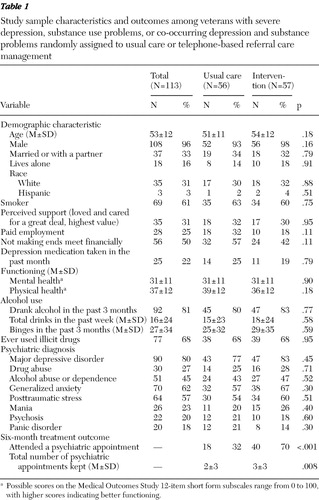 |
Forty patients had severe depression, 40 had substance use problems, and 33 had co-occurring disorders. Across the diagnostic groups significant characteristic differences indicated that the group with co-occurring disorders had the highest proportion of individuals who reported not making financial ends meet (22 patients, or 67%) ( χ2 =9, df=2, p=.01) and who met criteria for PTSD (25 patients, or 76%) ( χ2 =7, df=2, p=.03), compared with the depression group (difficult finances, 21 patients, or 53%; PTSD, 19 patients, or 48%) and the substance problems group (difficult finances, 13 patients, or 33%; PTSD, 20 patients, or 50%), respectively. The substance use disorder group had the lowest proportion of individuals meeting criteria for anxiety (N=19, or 48%) ( χ2 =6, df=2, p=.04) compared with the depression group (26 patients, or 65%) and the co-occurring disorders group (25 patients, or 76%). The group with substance use problems had higher levels of both mental health functioning (mean±SD score 36±13; F=6, df=2 and 95, p=.003) and physical health functioning (44±11; F=11, df=2 and 95, p=.001) compared with the comorbid group (mental function, 27±8; physical function, 35±10) and depression group (mental function, 29±9; physical function, 32±12), respectively (mental and physical health function scores range from 0 to 100, with higher scores representing better functioning).
Outcomes
Adherence to scheduled appointments. Participants in the intervention group were significantly more likely to attend their scheduled psychiatric appointment compared with the usual care group (70% versus 32%, respectively) ( χ2 =16, df=1, p<.001) ( Table 1 ). Follow-up analyses showed no diagnostic or age-group interaction effects on treatment attendance.
Total number of appointments attended. Overall, participants in the intervention groups attended significantly more psychiatric appointments over the six months (<3±3) compared with the usual care group (<2±3) (F=7, df=1 and 111, p=.008) ( Table 1 ). Follow-up analyses showed no diagnostic or age group interaction effects on the number of appointments attended. Further, the intervention effect remained significant when the analyses independently controlled for diagnostic and age group interaction effects. When the total number of appointments kept was compared among participants who attended at least one appointment, there was no significant difference between the intervention (4±2) and the usual care (4±3) groups.
Process outcomes. Among the intervention participants, 48 (84%) successfully completed the brief intervention (number of phone calls made=2±1, with a range of one to five phone calls), 18 (32%) required two TBR-CM sessions, and nine (16%) were unable to be contacted yet still received reminder letters and phone messages (mean number of calls made 5±2, with a range of two to six calls), significantly more than the group that successfully completed the brief intervention (F=37, df=1 and 55, p≤.001). Intervention participants who received the brief intervention were significantly more likely to attend their scheduled psychiatric appointment (38 participants, or 79%), compared with intervention participants who did not complete the brief intervention (two participants, or 22%) ( χ2 =15, df=1, p<.001).
Discussion
This investigation has provided evidence for the effectiveness of a clinical intervention aimed at overcoming the challenge of engaging primary care patients into specialty psychiatric care by improving motivation and reducing perceived barriers to treatment attendance. Data showed a twofold increase in psychiatric appointment attendance among patients randomly assigned to the intervention care group, which indicates that TBR-CM can be an effective option for initiating psychiatric treatment. Furthermore, the intervention effect did not differ across diagnostic or age groups, which demonstrates that this intervention can be generalizable across the represented diagnostic and age distributions. This study demonstrates the utility of expanding BMI and disease management techniques to improve adherence to referral psychiatric care. Earlier research has shown effectiveness in reducing psychiatric symptomatology ( 10 , 11 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ) when using such techniques but not specifically in improving psychiatric referral care. Further, the intervention by telephone replicated the efficiency and success of using telephone-based clinical management ( 2 , 29 ).
Patients who received TBR-CM were significantly more likely to attend their initial psychiatric appointment, and they were also more likely to have attended a greater number of psychiatric appointments over the six months after baseline. Further, intervention group patients who completed the intervention session were more likely to attend initial psychiatric appointments. The importance of improving initial treatment engagement was supported by evidence showing that once engaged into treatment, rates of continued attendance did not differ across randomization groups. As a result, TBR-CM functioned by improving the likelihood of attending the initial psychiatric appointment, as opposed to increasing the overall number of appointments. We would expect that psychiatric care engagement produces better health outcomes, because there are more opportunities to receive necessary treatment. However, this assumption needs to be fully evaluated through further research.
The efforts involved in the implementation of TBR-CM are important to highlight. TBR-CM implementation minimally entails one extensive phone session and one mailed letter. Our study, however, indicates that a range of one to six calls can be made, with a higher number of contacts made among patients who do not ultimately attend their treatment appointment. As a result, future studies may benefit by focusing efforts on patients who can be successfully contacted within two or three phone calls. However, such a limitation should be implemented with caution; hard-to-reach patients represent an important target group for intervention effectiveness. Further, the strict interpretation of the range of phone calls as representing effort is limiting, since total time spent on the phone was not recorded and phone calls without motivational enhancement have been known to not improve treatment adherence ( 1 , 12 , 30 ). For instance up to two calls made for the initial and follow-up sessions are expected to take approximately 30 minutes total. However, in the case that only messages are left and an intervention was not implemented, six calls should not require more than ten minutes on the phone total. In summary, future studies need to evaluate the effectiveness of implementing limited contacts as part of the intervention, record the total amount of effort (in minutes) involved in the intervention, and then evaluate whether the program benefits outweigh the fiscal costs of intervention delivery.
Although the TBR-CM intervention clearly increased engagement in psychiatric treatment in this sample, this study involved a limited sample consisting mostly of male veterans, motivated and committed to the research aims of the study. It is possible that these results will not generalize beyond this population, because veterans are a unique participant population and the PVAMC is a unique health care system. Further research is suggested to investigate whether TBR-CM is equally beneficial to the general population, specifically women, nonveterans, and individuals with psychiatric symptoms outside of depression and substance abuse. As a result, future studies need to evaluate TBR-CM effectiveness in a larger demographically diverse population. Also, further examination of study data may provide useful information on the specific participant characteristics for those where the intervention proved not successful, to identify intervention barriers when initiating referral management.
Conclusions
The TBR-CM referral management intervention increased attendance at the initial scheduled psychiatric appointment and increased the total number of psychiatric appointments attended during the following six months. TBR-CM can be tailored to a wide array of individuals, shown by the intervention's effectiveness across diagnostic and age groups. Overall, TBR-CM is a useful clinical service for helping patients initiate psychiatric treatment and is a program that can be incorporated into clinical care, specifically in an integrative care model such as the BHL.
Acknowledgments and disclosures
The authors gratefully acknowledge the enthusiastic cooperation of patients and staff involved in TBR-CM. This research was supported by a pilot research grant from the Department of Veterans Affairs (Veterans Integrated Services Network 4 Mental Illness Research Education and Clinical Center) and by training grant 5-T32-MH19931 from the National Institute of Mental Health.
The authors report no competing interests.
1. Lefforge NL, Donohue B, Strada MJ: Improving session attendance in mental health and substance abuse settings: a review of controlled studies. Behavioral Therapy 38:1–22, 2007Google Scholar
2. Oslin DW, Ross J, Sayers S, et al: Screening, assessment, and management of depression in VA primary care clinics: the Behavioral Health Laboratory. Journal of General Internal Medicine 21:46–50, 2006Google Scholar
3. Dobscha SK, Delucchi K, Young ML: Adherence with referrals for outpatient follow-up from a VA psychiatric emergency room. Community Mental Health Journal 35:451–458, 1999Google Scholar
4. Kerr EA, McGlynn EA, Adams J, et al: Profiling the quality of care in twelve communities: results from the CQI study. Health Affairs 23(3):247–256, 2004Google Scholar
5. Dawson DE, Grant BF, Stinson FS, et al: Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction 100:281–292, 2005Google Scholar
6. Cohen E, Feinn R, Arias A, et al: Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence 86:214–221, 2007Google Scholar
7. Huang B, Dawson DA, Stinson FS, et al: Prevalence, correlates, and comorbidity of nonmedical prescription drug use and drug use disorders in the United States: results of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry 67:1062–1073, 2006Google Scholar
8. Hattenschwiler J, Ruesch P, Modestin J: Comparison of four groups of substance-abusing in-patients with different comorbidity. Acta Psychiatrica Scandinavica 104: 59–65, 2001Google Scholar
9. Rollnick S, Miller WR: What is motivational interviewing? Behavioral and Cognitive Psychotherapy 23:325–334, 1995Google Scholar
10. Oslin DW, Sayers S, Ross J, et al: Disease management for depression and at-risk drinking via telephone in an older population of veterans. Psychosomatic Medicine 65:931–937, 2003Google Scholar
11. Felker BL, Chaney E, Rubenstein LV, et al: Developing effective collaboration between primary care and mental health providers. Journal of Clinical Psychiatry 8(Primary Care Companion suppl):12–16, 2006Google Scholar
12. Grupp-Phelan J, Delgado SV, Kelleher KJ: Failure of psychiatric referrals from pediatric emergency department. BMC Emergency Medicine 7:12–19, 2007Google Scholar
13. Cohen J: A power primer. Psychological Bulletin 112:155–159, 1992Google Scholar
14. Kroenke K, Spitzer RL, Williams JB: The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 16:606–613, 2001Google Scholar
15. Sheenhan DV, Lecrubier Y, Sheehan KH, et al: The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD 10. Journal of Clinical Psychiatry 59(20): 22–33, 1998Google Scholar
16. Sobell LC, Sobel MB: Timeline follow-back: a technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption. Edited by Litten R, Allen J. Totowa, NJ, Humana Press, 1992Google Scholar
17. Paykel ES, Myers JK, Lindenthal JJ, et al: Suicidal feelings in the general population: a prevalence study. British Journal of Psychiatry 124:460–469, 1974Google Scholar
18. Ware JE, Kosinski M, Keller SD: A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Medical Care 34:220–233, 1996Google Scholar
19. Zillmer EA, Fowler PC, Gutnick HN, et al: Comparison of two cognitive bedside screening instruments in nursing home residents: a factor analytic study. Journal of Gerontology 45(2):P69–P74, 1990Google Scholar
20. Moyer A, Finney JW, Swearingen CE, et al: Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction 97: 279–292, 2002Google Scholar
21. Ballesteros J, Duffy JC, Querejeta I, et al: Efficacy of brief interventions for hazardous drinkers in primary care: systematic review and meta-analyses. ACER 28:608– 618, 2004Google Scholar
22. Fleming MF, Manwell LB, Barry KL, et al: Brief physician advice for alcohol problems in older adults: a randomized community-based trial. Journal of Family Practice 48:378–384, 1999Google Scholar
23. Fleming MF, Mundt MP, French MT, et al: Brief physician advice for problem drinkers: long-term efficacy and benefit-cost analysis. Alcoholism: Clinical and Experimental Research 26(1):36–43, 2002Google Scholar
24. Blow FC, Barry KL: Older patients with at-risk and problem drinking patterns: new developments in brief interventions. Journal of Geriatric Psychiatry and Neurology 13(3):115–123, 2000Google Scholar
25. White HR, Mun EY, Pugh L, et al: Long-term effects of brief substance use interventions for mandated college students: sleeper effects of an in-person personal feedback intervention. Alcoholism Clinical Experimental Research 31:1380–1391, 2007Google Scholar
26. Carey KB, Carey MP, Maisto SA, et al: Brief motivational interventions for heavy college drinkers: a randomized controlled trial. Journal of Consulting and Clinical Psychology 74:943–954, 2006Google Scholar
27. Spooren D, Van Heeringen C, Jannes C: Strategies to increase compliance with out-patient aftercare among patients referred to a psychiatric emergency department: a multi-centre controlled intervention study. Psychological Medicine 28:949–956, 1998Google Scholar
28. Swanson AJ, Pantalon MV, Cohen KR: Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. Journal of Nervous and Mental Disease 187:630–635, 1999Google Scholar
29. McKay JR, Lynch KG, Shepard DS, et al: The effectiveness of telephone-based continuing care in the clinical management of alcohol and cocaine use disorders: 12-month outcomes. Journal of Consulting and Clinical Psychology 72:967–979, 2004Google Scholar
30. Shoffner J, Staudt M, Marcus S, et al: Using telephone reminders to increase attendance at psychiatric appointments: findings of a pilot study in rural Appalachia. Psychiatric Services 58:872–875, 2007Google Scholar



