Collaborative Care for Bipolar Disorder: Part I. Intervention and Implementation in a Randomized Effectiveness Trial
Coauthors for Cooperative Studies Program 430
The following individuals served as full coauthors for this article but could not be listed on the title page because of space limitations.
George Brown, M.D.
Mountain Home VAMC and Department of Psychiatry, Quillen College of Medicine, Johnson City, Tennessee
Steven G. Eilers, M.D.
Dallas VAMC and Department of Psychiatry, University of Texas Southwestern Medical School
Denise Evans, M.D.
Augusta VAMC and Department of Psychiatry, Medical College of Georgia, Augusta
Howard Fenn, M.D.
Palo Alto VAMC and Department of Psychiatry, Stanford University, Palo Alto, California
Richard Hauger, M.D.
San Diego VAMC and Department of Psychiatry, University of California, San Diego
Gail F. Kirk, M.S.
VA Cooperative Studies Coordinating Center, Perry Point, Maryland
Aimee Mayeda, M.D.
Indianapolis VAMC and Department of Psychiatry, Indiana University School of Medicine, Indianapolis
Janet Tekell, M.D.
Ann Arbor VAMC and Department of Psychiatry, University of Michigan, Ann Arbor
Hagop S. Akiskal, M.D.
San Diego VAMC and Department of Psychiatry, University of California, San Diego
Joseph F. Collins, Sc.D.
VA Cooperative Studies Coordinating Center, Perry Point, Maryland, and Department of Biostatistics, University of Maryland, College Park
Kousick Biswas, Ph.D.
VA Cooperative Studies Coordinating Center, Perry Point, Maryland
Pamela Staves, M.S., N.P.
Denver VAMC and VISN 19 MIRECC
Philip Lavori, Ph.D.
VA Cooperative Studies Coordinating Center and Department of Health, Research, and Policy, Stanford University, Palo Alto, California
Bipolar disorder is common—from 1 to 6 percent lifetime prevalence in the United States ( 1 , 2 )—and chronic. It is characterized by recurring manic and depressive symptoms and often psychosis ( 3 ). The disorder is associated with high suicide rates ( 4 ) and substantial social dysfunction ( 3 ), ranking sixth as a cause of disability worldwide ( 5 ). It may be the most expensive mental disorder for U.S. private behavioral health plans ( 6 ) and employers ( 7 ). Lifetime total costs per patient exceed $250,000 ( 8 ), with up to 70 percent of direct treatment costs generated outside the mental health sector ( 9 ).
As with other chronic medical illnesses, the cornerstone of managing bipolar disorder is evidence-based pharmacotherapy ( 10 , 11 ); however, undertreatment in ordinary clinical practice is an endemic problem ( 12 , 13 , 14 ). The President's New Freedom Commission report noted that fragmented care, suboptimal clinical outcomes, substantial functional deficits, and high costs characterize all severe and persistent mental illnesses, including bipolar disorder ( 15 ). The Institute of Medicine has recognized that these characteristics also describe other chronic medical illnesses ( 16 , 17 ).
Underscoring the problems associated with undertreatment, the low medication response rates in recent real-world clinical trials for depression ( 18 , 19 ), schizophrenia ( 20 ), and bipolar disorder ( 21 ) indicate that more comprehensive approaches for such illnesses are warranted. The development of structured psychotherapies for bipolar disorder, including cognitive-behavioral therapy ( 22 , 23 , 24 , 25 ), family therapy ( 26 ), and psychoeducation ( 27 , 28 ), is promising, although effectiveness appears limited for persons with greater impairment ( 25 ).
Moreover, promising treatments for chronic conditions do not easily move from initial clinical trials to general practice for mental illnesses ( 29 , 30 , 31 ) or other medical illnesses ( 16 , 32 , 33 ). The dearth of evidence-based interventions applicable for the public sector is particularly notable; less than 3 percent of mental health clinical trials have been conducted in such settings ( 34 ). How, then, can outcome be improved for individuals with bipolar disorder under real-world clinical conditions, particularly in the public sector?
In this article we summarize the conceptual background and development of an intervention model for the treatment of bipolar disorder, describe the design of a three-year, 11-site randomized effectiveness trial, and report data describing its successful implementation.
Intervention development
In 1992 we evaluated treatment needs for individuals with bipolar disorder at our Department of Veterans Affairs medical center (VAMC). Comorbidity was common, and the population tended to be poor, have chronic disabilities, and be without families or social support networks. Without funding to develop a mobile treatment team or wraparound services such as a program for assertive community treatment ( 35 , 36 , 37 ), we were limited to reorganizing existing outpatient clinic-based services.
Given the available literature on interventions for chronic illness, we recognized that our intervention would need to address three factors ( 38 ). First, the intervention should accommodate severely ill patients with comorbidities, who are common in clinical practice but typically excluded from clinical trials ( 39 ). Second, the intervention should minimize the provider-based variability typical of medical-surgical ( 40 , 41 , 42 ) and mental health ( 43 ) care. Evidence-based clinical practice guidelines could reduce such provider-based variability, but they have not been well implemented under naturalistic conditions ( 44 ). Third, the intervention should minimize the substantial system-related barriers characteristic of chronic care ( 32 , 33 , 45 , 46 ) to allow providers and patients to come together for timely, proactive illness management.
After a literature review, consultation with experts in bipolar disorder, and discussion with patients, we identified two main conceptual models ( 13 ). First, in the 1970s lithium clinics supported the transition from predominantly or exclusively psychotherapeutic treatment to a medical model of treatment for bipolar disorder ( 47 , 48 , 49 , 50 , 51 ). These clinics were organized around medication delivery, typically by a team consisting of a psychiatrist and support staff, with an emphasis on standardized care. Patient education was critical; provision of information was often supplemented by support groups to facilitate destigmatization, peer-based learning, and mutual support. Our specific orientation toward patient education derived from nursing practice, which has long emphasized patient education and collaborative decision making ( 52 , 53 ). The value of a collaborative patient-centered approach was underscored by the feedback we received from patients and has been documented recently in formal studies ( 54 , 55 ).
Second, we became aware of the chronic care models being developed for chronic medical illnesses by Wagner, Von Korff, and others ( 32 , 33 , 56 , 57 ). These models recognize that chronic illnesses are inadequately treated, despite the availability of efficacious medications. They emphasize anticipatory, patient-centered care by addressing four aspects of clinical care. Patient self-management skills are enhanced via education and collaborative goal setting. Provider decision making is supported by expert guidance, which may range from provision of practice guidelines to facilitated specialist consultation. Information flow is facilitated through various methods, ranging from development of complex electronic infrastructure to use of support staff to ensure that the clinician is provided with adequate patient data during the encounter. Work role redesign for both physician and support staff is typically required to achieve these changes.
The central focus of chronic care models, based on principles of social learning and self-regulation theories ( 56 ), is to reorganize medical care to support an effective partnership between clinicians and patients to improve outcomes relevant to patients ( 58 ). A recent review indicates that such interventions improved process or outcome measures in 32 of 39 clinical trials. All five trials that used all four components showed benefit. Notably, 19 of 20 interventions with a patient self-management component were effective ( 30 ).
We therefore defined the collaborative chronic care model as "an organization of care that emphasizes the patient's development of illness management skills and supports provider capability and availability in order to engage patients in timely, joint decision making about their illness" ( 13 ). The model articulates "chronic" care not in any pessimistic sense but rather to emphasize ongoing, anticipatory (rather than reactive, crisis-oriented) management ( 32 , 33 , 56 , 57 ). In fact, the strong emphasis on patient-centered collaboration anticipates current wellness and recovery orientations ( 55 ).
On the basis of these considerations, we organized a team-based intervention ( 57 ) that consisted of patient psychoeducation to improve self-management skills, simplified clinical practice guidelines, and use of a nurse care coordinator working in collaboration with a supervising psychiatrist to enhance continuity of care and information flow as described in detail below. We conducted a population-based, quasi-experimental study ( 59 ) with 103 veterans with bipolar disorder at our VAMC, excluding only those with dementia. Bipolar care was transferred to the intervention; no other existing care was changed, and specialty care referrals were made as clinically indicated. Compared with baseline, bipolar-specific pharmacotherapy increased without increased side effects, and patient satisfaction increased dramatically; the intervention retained more than 90 percent of participants at one year. Among those hospitalized in the prior year, psychiatric hospital days declined to 57 percent, and direct treatment costs were reduced by 65 percent.
On the basis of these data, in 1996 the VA Cooperative Studies Program (CSP) funded a three-year, multisite randomized controlled trial to test this model (CSP 430). Our hypotheses ( 38 ) were that, compared with usual care, the intervention would improve clinical outcome, with gains maximal over years 2 and 3; reduce total direct (mental health plus medical-surgical) treatment costs from the VA's economic perspective over three years; and improve functional outcome by the third year. The lag time to response would be consistent with preliminary data ( 59 , 60 ) and characteristic of social learning theory ( 56 ).
Cooperative Studies Program 430 Inclusion and Exclusion Criteria
Inclusion criteria
♦ Diagnosis of bipolar disorder type I or II by criteria on the Structured Clinical Interview for Axis I DSM-IV Disorders (68). All psychiatric and medical comorbidities were allowed except as specified below.
♦ Index episode of manic, major depressive, or mixed episode, by DSM-IV criteria, requiring hospitalization on an acute psychiatric ward
♦ At least two hospitalizations on acute psychiatric wards more than three months apart over the prior five years
Exclusion criteria
♦ Moderate to severe dementia, with a Mini-Mental State Examination score of ≤26 (69).
♦ Unresolved substance intoxication or withdrawal
♦ Hospitalization on chronic or acute psychiatric wards for six or more months in the past year
♦ Ongoing enrollment in mental health programs with a mobile outreach component in which clinical caregivers deliver services to the patient in the community
♦ Terminal medical illness with less than three years of expected longevity
♦ Unable or unwilling to give informed consent or in other ways unable to complete study requirements
♦ Participation in another concurrent experimental mental health or medical-surgical treatment protocol
Effectiveness trial design
Emphasizing effectiveness
Intervention effects commonly are attenuated when moving from initial testing by expert hands in highly selected samples to testing with other clinicians and less restrictive samples ( 16 , 17 , 38 , 45 , 61 , 62 , 63 , 64 ). The initial efficacy approach emphasizes internal validity of the trial to isolate treatment effects under ideal conditions. The latter effectiveness approach, like practical clinical trials ( 65 , 66 ), emphasizes external validity—the applicability of results to the settings in which the intervention will be applied. Unfortunately, only a minority of mental health clinical trials adequately address external validity characteristics ( 67 ). Accordingly, the study design committee emphasized such effectiveness characteristics from the outset, addressing four interrelated aspects of protocol design: sample, intervention, assessment methods, and data analysis ( 38 ).
Sample
A high priority was to recruit a sample that would resemble typical public-sector patients, particularly those receiving care in the VA system. We thus required a DSM-IV diagnosis of bipolar disorder via structured interview ( 68 ) but employed broad inclusion and minimal exclusion criteria, including those with comorbid illnesses and excluding only those for whom the protocol would not be feasible or relevant, such as patients with dementia ( 69 ) (see box on the next page). Potential participants were identified during acute hospitalization for bipolar disorder and randomly assigned at discharge to either continue usual outpatient care or receive care in the intervention clinic for three years. Randomization was stratified on the basis of receipt of living support services (group homes, for example).
Site recruitment is frequently not reported in clinical trials but is an important determinant of external validity ( 45 ). We solicited participation from all VAMCs through their research offices. We established two criteria for site selection: VAMCs must have 24-hour emergency service access, and they must not be in the bottom quartile nationally of mental health visits per patient per year. Following these criteria ensured that we would not be comparing the intervention with substandard care while allowing us to draw from a heterogeneous group of VAMCs. Among 50 responses, 12 VAMCs were chosen to ensure diversity of geographic location, urban as well as rural location, size, mission (historically general as well as neuropsychiatric), and prior research productivity. The intervention development site ( 59 ) was excluded. One site (large, urban, high research productivity) and its data were dropped at midstudy because of irregularities in data collection.
Intervention
The Bipolar Disorders Program intervention ( 13 , 38 , 59 ) consists of an outpatient clinic "specialty team" of a psychiatrist and a nurse care coordinator ( 57 ). Specifically, staffing requires a .5 full-time-equivalent (FTE) nurse and a .25 FTE psychiatrist for 45 to 50 patients. (We included in this caseload additional patients with bipolar-spectrum disorders who were not randomly assigned to the intervention.) The Bipolar Disorders Program is situated in the mental health outpatient clinic, without after-hours availability or mobile community outreach. All bipolar-specific care is provided in the intervention, but no other care is changed. For example, specialty mental health treatment (psychotherapy and substance treatment) is continued, and other referrals are made if clinically indicated. Similarly, enrollment in primary care and collaboration with medical providers are emphasized.
Three intervention components address patient, provider, and system aspects of care. Each component is specified in a detailed manual (available from the first author).
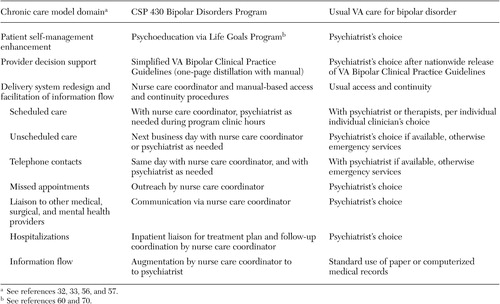
To enhance illness self-management skills, the Bipolar Disorders Program nurse care coordinator enrolled participants in group psychoeducation in the first months of care in the intervention. This Life Goals Program stresses identification of personal symptom profiles, early warning symptoms, and triggers. It uses "personal cost-benefit analyses" and group feedback to improve coping responses and develop collaborative action plans with providers ( 60 , 70 , 71 ). The Life Goals Program goes beyond one-way information transfer and stimulates active self-management and collaborative activities ( 13 , 58 ).
Simplified VA Bipolar Clinical Practice Guidelines ( 72 ) offer expert guidance to providers for decision making. We reasoned that access to specialty consultants (often called "collaborative care" or "stepped care" in primary care studies) would not regularly be available at all VAMCs. We thus decided to rely on published guidelines that were distilled to a single reference algorithm and six-page manual ( 13 ). The algorithm focuses on the endemic underrecognition and undertreatment of mood episodes ( 12 , 13 , 14 ) and stresses identification of episodes and subsyndromal symptoms and their aggressive treatment. The algorithm specifies classes of medications to use (for example, antimanics and antidepressants) rather than sequencing individual agents (such as lithium or paroxetine) to allow for patient-centered collaborative decision making that is based on efficacy and side effects. Preliminary data indicated that the algorithm increased bipolar-specific pharmacotherapy across four VAMCs ( 13 ). The practice guidelines were updated throughout the study as new medications demonstrated efficacy for bipolar disorder.
System reorganization to improve access to and continuity of care and information flow is implemented by using a nurse care coordinator to augment the psychiatrist's effort. The nurse's access and continuity manual considers three types of contacts. "Backbone scheduled care" consists of regularly scheduled appointments for monitoring the patient regardless of clinical status. "Demand-responsive services" are requested by patients for issues that cannot wait until the next scheduled appointment—for example, to alleviate side effects, address nonresponse to a change in medication, or help with crisis management. Nurse care coordinators provide same-day telephone response and next-business-day clinic visits on demand, similar to what are now called "open access" clinics ( 73 ). "Outreach and inreach contacts" include, respectively, aggressive follow-up for missed appointments and liaison with other providers during admissions and emergency room visits or for care coordination. These activities involve collaborating with mental health and medical-surgical providers (concerning, for example, substance relapse, hypertension, obesity, and confusion over medical medications). Nurse care coordinators also facilitate information flow to the psychiatrist by providing patient assessments, implementing reminders for guideline-based monitoring, and tracking laboratory values. We took this low-technology approach to information flow—one not dependent on electronic medical record or specialized information-processing technology—so that the intervention could be disseminated even to small non-VA sites.
Consistent with our effectiveness orientation, we balanced the need for intervention fidelity (internal validity) with training and monitoring that would be typical of other VA specialty programs (external validity) ( 13 ). We conducted two-day clinical training at the start of the study. During the study, new nurses completed one-day on-site training, and psychiatrists received telephone training. Nurses were trained to criterion and monitored in the Life Goals Program ( 60 ). Regular conference calls and newsletters provided updates on treatment guidelines, aided discussion of difficult cases, and reviewed access and continuity issues.
Fidelity monitoring avoided intense scrutiny of practice style (such as chart reviews or audiotaping) and relied instead on continuous quality improvement methods using audit-feedback monitoring ( 74 , 75 ). Three parameters were chosen: caseload, completion of phase I of the Life Goals Program, and the "critical service encounter" index. Each Bipolar Disorders Program was expected to maintain a caseload of 45 to 50 patients (including patients with bipolar disorder who were randomly selected for the intervention plus others who were not part of the protocol). Participants were to complete phase I of the Life Goals training within the first 12 months of enrollment in the intervention. The critical service encounter index was constructed as follows to measure access to care and continuity of care. Ideally, all unscheduled mental health contacts ("critical service encounters") should be with a Bipolar Disorders Program or other ongoing mental health provider, rather than with an emergency room, medication refill clinic, or other triage visit. The critical service encounter index was therefore calculated by dividing the number of unscheduled contacts outside of the Bipolar Disorders Program or other ongoing providers by all unscheduled contacts (including unscheduled contacts with intervention staff or other ongoing providers). We expected that the critical service encounter index would remain below 10 percent. Monitoring data were fed back to the sites in monthly newsletters and conference calls.
Usual care
Participants who were randomly assigned to usual care continued with their previous psychiatrist or were assigned one if new to the VA. Clinicians caring for usual-care participants and clinicians for the intervention each received intake data according to the Structured Clinical Interview for DSM-IV. No intervention clinicians cared for participants enrolled in usual care. To avoid a Hawthorne effect (inducing a change in behavior simply by monitoring), no monitoring of usual care was undertaken. However, process parameters for evaluating characteristics of usual care (for example, collaborative practice style and number of ambulatory visits) were collected for secondary analyses.
Intervention components are compared with usual care in Table 1 . Three clinical vignettes are available as online supplements to this article to qualitatively illustrate participation in the intervention (see ps.psychiatryonline.org).
 |
Assessment
The effectiveness orientation requires outcome assessment with minimal respondent burden, preferably with data that can be provided by proxy where necessary ( 38 ). The outcome battery was administered in 45 to 75 minutes every eight weeks and covered clinical and functional outcome, quality of life, non-VA clinical service use, and selected process measures as detailed in a companion article in this issue of Psychiatric Services ( 76 ). Preliminary work indicated that completion of the assessment was tolerated well, with over 90 percent of desired data collected and high correlations between participant data and proxy data ( 77 ).
Because participants could not be blinded to the intervention, we could not guarantee blinding of the research assistants. However, assessments were scripted, and interviewers were trained to criterion and reassessed regularly ( 38 ). Furthermore, a "firewall" was established between research assistants and clinicians to allow communication of participant information only in situations of acute danger to the participant or to others. This prevented clinical information from biasing research evaluations (internal validity) and prevented the research assistant from inadvertently acting as a case manager, which would not be part of the intervention when disseminated (external validity).
Cost data were collected from the economic perspective of the VA. Perspective in cost analyses indicates whose dollars are being counted ( 78 ). Our primary cost hypothesis focused on costs to the payer, the VA (rather than all societal costs). Thus all treatment costs (inpatient, outpatient, pharmacy, other; mental health, medical-surgical, other) were included; lost wages and other indirect costs were not. Over 90 percent of service contacts in our preliminary study were within the VA system ( 59 ), so we used the VA National Patient Care Database and Pharmacy Benefits Management Package (www.virec.research. med.va.gov/datasourcesname/datanames.htm) to identify patient-specific costs. This source of data was supplemented by participant report of non-VA service use at bimonthly interviews, an interval for which recall remains intact ( 59 ).
Data analysis
Data-analytic approaches in an effectiveness trial must reflect real-world considerations of treatment effects and be able to deal with heterogeneous individuals who may periodically be lost to follow-up ( 38 ). Although analytic techniques are described in detail in the companion to this article ( 76 ), note here that our a priori hypotheses aimed to help detect change over time rather than acutely, as medical chronic care investigators have done ( 79 ). Thus our focus was long-term "illness load" ( 77 , 80 ) rather than time to remission or time to first relapse, and statistical techniques were designed to handle intermittent missing data with mixed-effects models.
How successfully was the effectiveness trial implemented?
Sample
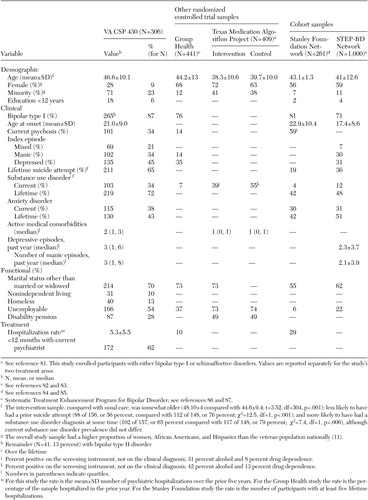
The protocol randomly assigned 330 participants, and outcome data were collected for 306 (93 percent). Flow of participants throughout the study is illustrated in Figure 1 according to CONSORT (Consolidated Standards of Reporting Trials) guidelines, and participant characteristics are summarized in Table 2 . As can be seen, in comparison with other large samples of patients with bipolar disorder ( 81 , 82 , 83 , 84 , 85 , 86 , 87 ), our sample was somewhat older, severely ill, and highly complex. Participants had high rates of hospitalization, prior suicide attempts, substance and anxiety disorders, and active medical illnesses. Most were without families and unemployable, and 13 percent were homeless. The proportion of women and minorities matched or exceeded rates in the veteran population ( 38 ).

a Per CONSORT (Consolidated Standards of Reporting Trials) guidelines. For the study, 330 participants were randomly assigned, which represents 12 percent of those screened and 27 percent of those meeting inclusion and exclusion criteria (330 of 1,231). Participants did not meet inclusion criteria if they did not meet DSM criteria for bipolar disorder or current episode requiring admission to an acute psychiatric ward (N=597) or if they did not meet prior hospitalization criteria of at least two acute psychiatric hospitalizations separated by at least three months over the prior five years (N=902). Patients did not meet exclusion criteria if they had a Mini-Mental State Examination score below 26 (N=20), could not be reevaluated after substance detoxification (N=31), were hospitalized for more than six months over the prior year (N=18), were treated in community or mobile mental health programs (N=28), had terminal illness with less than three years' life expectancy (N=16), were unable to give informed consent (N=115), or were enrolled in other research programs (N=32). Stratification was based on whether the participant resided in an assisted living facility (high stratum) or lived independently (low stratum). Analyses were based on data from 306 participants.
 |
Intervention
Fidelity monitors indicated excellent implementation of the intervention, including the median monthly caseload of 47 (quartiles 41 and 48) and Life Goals Program completion by one year for 78 percent of the sample (quartiles 74 and 82). The critical service encounter index (see above for calculation) was 8 percent (quartiles 8 and 11); that is, 92 percent of unscheduled care was provided by the intervention or other ongoing clinicians, indicating excellent access and continuity.
Staff turnover during study years (1997 through 2003) resulted in 17 nurses and 25 psychiatrists staffing the 11 Bipolar Disorders Programs. Thus the intervention was well implemented despite typical staff turnover, suggesting that trial results reflect principles of treatment rather than skills of a small number of devotees.
Assessment
Despite the complexity of the population we studied, intake diagnostic reliability was excellent for mood, anxiety, and substance diagnoses, including differentiation of bipolar from substance-induced symptoms (Cramér's V=.91 to 1.00). Follow-up clinical and functional measures had similarly high interrater reliability (intra-class correlations of .82 to .91).
Conclusions
The Bipolar Disorders Program intervention is a collaborative chronic care model with conceptual roots in lithium clinics and medical chronic care models. The program provides a highly specified, manual-based intervention. However, unlike typical efficacy interventions, which are designed for maximal effect in select samples with less regard for complexity or cost, this intervention was developed for eventual dissemination. The randomized controlled trial was designed from an effectiveness perspective to maximize the likelihood that trial results would resemble those seen when disseminated. This developmental strategy anticipated subsequent arguments that interventions should be developed from the outset with consideration for their eventual dissemination ( 45 ).
The intervention was then tested in a multisite trial that emphasized effectiveness aspects to maximize generalizability of results. The trial recruited a complex sample typical of the population treated in VAMCs and successfully assessed participants over a three-year period with a lowburden assessment battery. The companion article in this issue ( 76 ) reports the results of this clinical trial.
Acknowledgments
This study was funded by Department of Veterans Affairs Cooperative Studies Program 430. The authors especially thank Nancy M. Shea, R.N., M.S., Tracy Wyrostek, Christina Moniz, Tara Burke, Erinn Dawson, M.A., Eileen Richardson, and G. F. Kimpton for their assistance. Additional acknowledgments are available in the online supplement to this article at ps.psychiatryonline.org. Some of the findings in this article were presented at the annual meeting of the American College of Neuropsychopharmacology, held in San Juan, Puerto Rico, December 12-16, 2004, and at the 18th Mental Health Services Research Conference, held by the National Institute of Mental Health in Washington, D.C., July 18-19, 2005.
1. Judd LL, Akiskal HS: The prevalence and disability of bipolar spectrum disorders in the US population. Journal of Affective Disorders 73:123-131, 2003Google Scholar
2. Hirschfeld RM, Calabrese JR, Weissman MM, et al: Screening for bipolar disorder in the community. Journal of Clinical Psychiatry 64:53-59, 2003Google Scholar
3. Bauer MS: Bipolar (manic-depressive) disorder, in Psychiatry, 2nd ed. Edited by Tasman A, Kay J, Lieberman J. Philadelphia, Saunders, 2003Google Scholar
4. Baldessarini RJ, Tondo L, Hennen J: Treating the suicidal patient with bipolar disorder: reducing suicide risk with lithium. Annals of the New York Academy of Science 932:24-43, 2001Google Scholar
5. Lopez AD, Murray CJL: Global mortality, disability, and the contribution of risk factors: the Global Burden of Disease Study. Nature Medicine 4:1241-1243, 1998Google Scholar
6. Peele PB, Xu Y, Kupfer DJ: Insurance expenditures on bipolar disorder. American Journal of Psychiatry 160:1286-1290, 2003Google Scholar
7. Goetzel RZ, Hawkins K, Ozminkowski RJ, et al: The health and productivity cost burden of the "Top 10" physical and mental health conditions affecting six large US employers in 1999. Journal of Occupational and Environmental Medicine 45:5-14, 2003Google Scholar
8. Begley CE, Annegers JF, Swann AC, et al: The lifetime cost of bipolar disorder in the US: an estimate for new cases in 1998. Pharmacoeconomics 19:483-495, 2001Google Scholar
9. Bryant-Comstock L, Stender M, Devercelli G: Health care utilization and cost among privately insured patients with bipolar I disorder. Bipolar Disorders 4:398-405, 2002Google Scholar
10. American Psychiatric Association: Practice guideline for the treatment of patients with bipolar disorder. American Journal of Psychiatry 159(Apr suppl):S1-S50, 2002Google Scholar
11. Bauer MS, Mitchner L: What is a "mood stabilizer"? An evidence-based response. American Journal of Psychiatry 161:3-18, 2004Google Scholar
12. Keller MB, Lavori PW, Klerman GL, et al: Low levels and lack of predictors of somatotherapy and psychotherapy received by depressed patients. Archives of General Psychiatry 43:458-466, 1986Google Scholar
13. Bauer MS: The collaborative practice model for bipolar disorder: design and implementation in a multi-site randomized controlled trial. Bipolar Disorders 3:233-244, 2001Google Scholar
14. Dickson W, Kendell R: Does maintenance lithium prevent recurrences of mania under ordinary clinical conditions? Psychological Medicine 16:521-530, 1986Google Scholar
15. Hogan MF: The President's New Freedom Commission: recommendations to transform mental health care in America. Psychiatric Services 54:1467-1474, 2003Google Scholar
16. Institute of Medicine: Assessing Medical Technologies. Washington, DC, National Academy Press, 1985Google Scholar
17. Committee on Quality Health Care in America: Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC, National Academy Press, 2001Google Scholar
18. Rush AJ, Trivedi MH, Wisniewski SR, et al: Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. New England Journal of Medicine 354:1231-1242, 2006Google Scholar
19. Trivedi MH, Fava M, Wisniewski SR, et al: Medication augmentation after the failure of SSRIs for depression. New England Journal of Medicine 354:1243-1252, 2006Google Scholar
20. Lieberman JA, Stroup TS, McEvoy JP, et al: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209-1223, 2006Google Scholar
21. Nierenberg AA, Ostacher MJ, Calabrese JR, et al: Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. American Journal of Psychiatry 163:210-216, 2006Google Scholar
22. Lam DH, Watkins ER, Hayward P, et al: A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder. Archives of General Psychiatry 60:145-152, 2003Google Scholar
23. Lam DH, Hayward P, Watkins ER, et al: Relapse prevention in patients with bipolar disorder: cognitive therapy outcome after 2 years. American Journal of Psychiatry 162:324-329, 2005Google Scholar
24. Ball JR, Mitchell PB, Corry JC, et al: A randomized controlled trial of cognitive therapy for bipolar disorder: focus on long-term change. Journal of Clinical Psychiatry 67:277-286, 2006Google Scholar
25. Scott J, Paykel E, Morriss R, et al: Cognitive-behavioural therapy for severe and recurrent bipolar disorders: a randomized controlled trial. British Journal of Psychiatry 188:313-320, 2006Google Scholar
26. Miklowitz DJ, George EL, Richards JA, et al: A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Archives of General Psychiatry 60:904-912, 2003Google Scholar
27. Perry A, Tarrier N, Morriss R, et al: Randomised controlled trial of efficacy of teaching patients with bipolar disorder to identify early symptoms of relapse and obtain treatment. British Medical Journal 318:149-153, 1999Google Scholar
28. Colom F, Vieta E, Martinez-Aran A, et al: A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Archives of General Psychiatry 60:402-407, 2003Google Scholar
29. Deci PA, Santos AB, Hiott DW, et al: Dissemination of assertive community treatment programs. Psychiatric Services 46:676-678, 1995Google Scholar
30. Phillips SD, Burns BJ, Edgar ER, et al: Moving assertive community treatment into standard practice. Psychiatric Services 52:771-779, 2001Google Scholar
31. Schoenwald SK, Hoagwood K: Effectiveness, transportability, and dissemination of interventions. Psychiatric Services 52:1190-1197, 2001Google Scholar
32. Bodenheimer T, Wagner EH, Grumbach K: Improving primary care for patients with chronic illness, part 1. JAMA 288:1775-1779, 2002Google Scholar
33. Bodenheimer T, Wagner EH, Grumbach K: Improving primary care for patients with chronic illness, the chronic care model, part 2. JAMA 288:1909-1914, 2002Google Scholar
34. Shumway M, Sentell TL: An examination of leading mental health journals for evidence to inform evidence-based practice. Psychiatric Services 55:649-653, 2004Google Scholar
35. Burns BJ, Santos AB: Assertive community treatment: an update of randomized trials. Psychiatric Services 46:669-675, 1995Google Scholar
36. Rosenheck RA, Neale M: Cost-effectiveness of intensive psychiatric community care for high users of inpatient services. Archives of General Psychiatry 55:459-466, 1998Google Scholar
37. Salkever D, Domino ME, Burns BJ, et al: Assertive community treatment for people with severe mental illness: the effect on hospital use and costs. Health Services Research 34:577-601, 1999Google Scholar
38. Bauer MS, Williford W, Dawson E, et al: Principles of effectiveness trials and their implementation in VA Cooperative Study #430, "Reducing the Efficacy-Effectiveness Gap in Bipolar Disorder." Journal of Affective Disorders 67:61-78, 2001Google Scholar
39. Zimmerman M, Chelminski I, Posternak MA: Generalizability of antidepressant efficacy trials: differences between depressed psychiatric outpatients who would or would not qualify for an efficacy trial. American Journal of Psychiatry 162:1370-1372, 2005Google Scholar
40. Wennberg J, Gittleshon A: Small area variations in health care delivery. Science 18:1102-1108, 1973Google Scholar
41. Chassin M, Brock R, Park R, et al: Regional variations in the use of common surgical services by the Medicare population. New England Journal of Medicine 314:285-290, 1986Google Scholar
42. Sui A, Jonnenberg F, Manning W: Inappropriate use of hospitals in a randomized trial of health insurance plans. New England Journal of Medicine 315:1259-1266, 1986Google Scholar
43. Fortney J, Booth B, Smith GR: Variations among VA hospitals in length of stay for treatment of depression. Psychiatric Services 47:608-613, 1996Google Scholar
44. Bauer MS: Quantitative adherence studies of mental health clinical practice guidelines. Harvard Review of Psychiatry 10:138-153, 2002Google Scholar
45. Glasgow RE, Lichtenstein E, Marcus AC: Why don't we see more translation of health promotion research to practice? Re-thinking the efficacy-to-effectiveness transition. American Journal of Public Health 93:1261-1267, 2003Google Scholar
46. Bauer MS, Williford WO, McBride L, et al: Perceived barriers to health care access in a treated population. International Journal of Psychiatry in Medicine 35:13-26, 2005Google Scholar
47. Shelley EM, Fieve RR: The use of non-physicians in a health maintenance program for affective disorders. Hospital and Community Psychiatry 25:303-305, 1974Google Scholar
48. Fieve RR: The lithium clinic: a new model for the delivery of psychiatric services. American Journal of Psychiatry 132:1018-1022, 1975Google Scholar
49. Gitlin MJ, Jamison KR: Lithium clinics: theory and practice. Hospital and Community Psychiatry 35:363-368, 1984Google Scholar
50. Foelker GA Jr, Molinari V, Marmion JJ, et al: Lithium groups and elderly bipolar outpatients. Clinical Gerontologist 5:297-307, 1986Google Scholar
51. Seeger PA, Stern SL, Dennert JW: A combined group and individual approach to outpatient lithium treatment. Ohio Medicine 85:300-302, 1989Google Scholar
52. Orlando L: The Dynamic Nurse Patient Relationship: Function, Process, and Principles. New York, Putnam, 1961Google Scholar
53. Wilkinson L: A collaborative model: ambulatory pharmacotherapy for chronic psychiatric patients. Journal of Psychosocial Nursing 29:26-29, 1991Google Scholar
54. Ludman EJ, Simon GE, Rutter CJ, et al: Adaptation of a measure for assessing patient perception of provider support for self-management of bipolar disorder. Bipolar Disorders 4:249-253, 2002Google Scholar
55. Lewis L: Patient perspectives on the diagnosis, treatment, and management of bipolar disorder. Bipolar Disorders 7(suppl 1):33-37, 2005Google Scholar
56. Von Korff M, Gruman J, Schaefer J, et al: Collaborative management of chronic illness. Annals of Internal Medicine 127:1097-1102, 1997Google Scholar
57. Wagner EH: The role of patient care teams in chronic disease management. British Medical Journal 320:569-572, 2000Google Scholar
58. Bodenheimer T, Lorig K, Holman H, et al: Patient self-management of chronic disease in primary care. JAMA 288:2469-2475, 2002Google Scholar
59. Bauer MS, McBride L, Shea N, et al: Impact of an easy-access VA clinic-based program for patients with bipolar disorder. Psychiatric Services 48:491-496, 1997Google Scholar
60. Bauer MS, McBride L, Chase C, et al: Manual-based group psychotherapy for bipolar disorder: a feasibility study. Journal of Clinical Psychiatry 59:449-455, 1998Google Scholar
61. Greenwald P, Cullen JW: The new emphasis in cancer control. Journal of the National Cancer Institute 74:543-551, 1985Google Scholar
62. Flay BR: Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs. Preventive Medicine 15:451-474, 1986Google Scholar
63. Clark GN: Improving the transition from basic efficacy research to effectiveness studies: methodological issues and procedures. Journal of Consulting and Clinical Psychology 63:718-725, 1995Google Scholar
64. Wells K: Treatment research at the crossroads: the scientific interface of clinical trials and effectiveness research. American Journal of Psychiatry 156:5-10, 1999Google Scholar
65. Tunis SR, Stryer DB, Clancy CM: Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 290:1624-1632, 2003Google Scholar
66. March JS, Silva SG, Compton S, et al: The case for practical clinical trials in psychiatry. American Journal of Psychiatry 162:836-846, 2005Google Scholar
67. Braslow JT, Duan N, Starks SL, et al: Generalizability of studies on mental health treatment and outcomes, 1981-1996. Psychiatric Services 56:1261-1268, 2005Google Scholar
68. First MB, Spitzer RL, Gibbon M, et al: Structured Clinical Interview for Axis I DSM-IV Disorders—(SCID). Patient ed, version 2.0. New York, Biometrics Research Department, New York Psychiatric Institute, 1996Google Scholar
69. Folstein M, Folstein S, McHugh P: "Mini-Mental State": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12:189-198, 1975Google Scholar
70. Bauer MS, McBride L: Structured Group Psychotherapy for Bipolar Disorder. New York, Springer, 1996Google Scholar
71. Yatham LN, Kennedy SH, O'Donovan C, et al: Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disorders 7(suppl 3):5-69, 2005Google Scholar
72. Bauer MS, Callahan A, Jampala C, et al: Clinical practice guidelines for bipolar disorder from the Department of Veterans Affairs. Journal of Clinical Psychiatry 60:9-21, 1999Google Scholar
73. Schall MW, Duffy T, Krishnamurthy A, et al: Improving patient access to the Veterans Health Administration's primary care and specialty clinics. Joint Commission Journal on Quality and Safety 30:415-424, 2004Google Scholar
74. Berwick DM, Godfrey AB, Roessner J: Curing Health Care: New Strategies for Quality Improvement. San Francisco, Jossey-Bass, 1990Google Scholar
75. Primer on Indicator Development and Application: Measuring Quality in Health Care. Chicago, Joint Commission on Accreditation of Healthcare Organizations, 1990Google Scholar
76. Bauer MS, McBride L, Williford WO, et al: Collaborative care for bipolar disorder: part II. impact on clinical outcome, function, and costs. Psychiatric Services 57:937-945, 2006Google Scholar
77. Bauer MS, Kirk G, Gavin C, et al: Determinants of functional outcome and health care costs in bipolar disorder: a high-intensity follow-up study. Journal of Affective Disorders 65:231-241, 2001Google Scholar
78. Gold MR, Siegel JE, Russell LB, et al: Cost-Effectiveness in Health and Medicine. New York, Oxford University Press, 1996Google Scholar
79. Katon W, Von Korff M, Lin E, et al: Collaborative management to achieve treatment guidelines. JAMA 273:1026-1031, 1995Google Scholar
80. Berghofer A, Müller-Oerlinghausen B: Is there a loss of efficacy of lithium in patients treated for over 20 years? Neuropsychobiology 42:S46-S49, 2000Google Scholar
81. Suppes T, Rush JA, Dennehy EB, et al: Texas Medication Algorithm Project: clinical results for patients with a history of mania. Journal of Clinical Psychiatry 64:370-382, 2003Google Scholar
82. Simon GE, Ludman EJ, Unützer J, et al: Design and implementation of a randomized trial evaluating systematic care for bipolar disorder. Bipolar Disorders 4:226-236, 2002Google Scholar
83. Simon GE, Ludman EJ, Bauer MS, et al: Long-term effectiveness and cost of a systematic care management program for bipolar disorder. Archives of General Psychiatry 63:500-508, 2006Google Scholar
84. McElroy SL, Altshuler LL, Suppes T, et al: Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. American Journal of Psychiatry 158:420-426, 2001Google Scholar
85. Suppes T, Leverich GS, Keck PE, et al: The Stanley Foundation Bipolar Treatment Outcome Network: II. demographics and illness characteristics of the first 261 patients. Journal of Affective Disorders 67:45-59, 2001Google Scholar
86. Kogan JN, Otto MW, Bauer MS, et al: STEP-BD investigators: demographic and diagnostic characteristics of the first 1000 patients enrolled in the Systematic Treatment Enhancement Program for Bipolar Disorders (STEP-BD). Bipolar Disorders 6:460-469, 2004Google Scholar
87. Simon NM, Otto MW, Wisniewski SR, et al: Anxiety disorder comorbidity in bipolar disorder. American Journal of Psychiatry 161:2222-2229, 2004Google Scholar



