Psychiatric Decision Making in the Adoption of a New Antipsychotic in Germany
Studies from other medical fields have demonstrated a long delay in the implementation of new research findings or in the use of new drugs, leading to suboptimal treatment of patients ( 1 ). However, the early uptake of unnecessary or ineffective new therapies is criticized as causing a financial burden on health systems.
It has been shown that individual physicians differ strongly as to how they adopt new strategies and how rapidly they implement them. The speed at which the physicians take up innovative new drugs depends not only on drug characteristics but also on physician characteristics ( 2 , 3 , 4 ).
From a political viewpoint, the challenge is to promote the uptake of innovations that have been shown to be effective, to delay the spread of those that have not yet been shown to be effective, and to prevent the uptake of ineffective innovations ( 1 ).
In mental health there is currently a heated discussion about the use of the second-generation antipsychotics. On the one hand, their widespread use is urgently advocated for and supported by data that suggest advantages relating not only to fewer extrapyramidal side effects but also to improvements in, for example, negative symptoms, cognitive functioning, and subjective well-being ( 5 , 6 ). On the other hand, these advantages are challenged by findings that tend to support therapeutic equivalence of the cheaper first-generation antipsychotics ( 7 , 8 , 9 ).
Information on how psychiatrists adopt new drugs and which factors influence their decisions is scarce, because research in the field of medical decision making has made only limited inroads into psychiatry ( 10 ). It was thus our aim to perform an explorative study of psychiatrists' adoption of a new drug.
Methods
Three months before and three months after the launch of the antipsychotic agent aripiprazole (Abilify) in June 2004, we conducted semistructured interviews with 50 hospital psychiatrists in Southern Germany. Aripiprazole was chosen as the drug to be studied because it was the next antipsychotic to be introduced on the German market and because it was announced as a "third-generation" antipsychotic, with a new mechanism of action, partial dopamine agonism. To recruit psychiatrists, we contacted five psychiatric hospitals: one university hospital and four state hospitals. The survey was introduced to the psychiatrists at morning rounds as a study about antipsychotic drug choice (without naming aripiprazole), and psychiatrists currently treating patients with schizophrenia were included in the survey.
The first interviews were undertaken in March or April 2004. Aripiprazole was launched in June 2004, and the second interviews were conducted in September 2004.
In conducting the interviews we were interested in the following topics. Which sources of information do the psychiatrists use in general? The frequency of use of different sources of information was measured by 5-point rating scales. Possible scores range from 1, never, to 5, regularly. Are the psychiatrists aware of the forthcoming introduction of the new drug? ("Have you heard or read about a drug called aripiprazole, or Abilify, prior to this interview?") Where did they learn about the new product? ("On which occasion did you first hear about the new drug?") How do they perceive statements on aripiprazole's efficacy, tolerability, and mechanism of action (as stated by the manufacturer)? Are the statements credible? How important are the stated efficacy, tolerability, and mechanism of action to their clinical work? Which patients do they intend to give this agent to, and which patients do they not? (From their currently treated patients, psychiatrists were requested to provide sociodemographic data, information on current medication, and scores on the Clinical Global Impression scale for the patients most likely and least likely be given aripiprazole.) How many psychiatrists used the new agent three months after introduction? What variables influenced these patterns?
Additionally, sociodemographic information about the psychiatrists was obtained, and the psychiatrists filled in a questionnaire, Ungewissheitstoleranzskala (UGTS), which assessed their uncertainty tolerance ( 11 ). The UGTS is an 8-item self-report questionnaire devised to measure a person's assessment of ambiguous situations. The UGTS showed satisfactory internal consistency (α=.72). It has been shown that individuals can be distinguished according to their tolerance of uncertainty. Previous research has demonstrated that patients with a high uncertainty tolerance are more likely to search for new health-related information ( 11 ). However, this measure has not been used in the context of psychiatrists' decision making. We hypothesized that psychiatrists with a high uncertainty tolerance would be more likely to adopt the new drug within the first three months after its introduction.
We performed an exploratory analysis of the data. Frequency statistics, nonparametric tests, and linear regression analysis were applied. A p value of <.05 was considered significant.
Results
Fifty psychiatrists from five psychiatric hospitals participated in the survey; 47 were surveyed at follow-up. Thirty-nine (78 percent) were men, and 11 (22 percent) were women. Thirty-three (66 percent) were residents, 16 (32 percent) were senior psychiatrists, and one (2 percent) was the department head. Their mean±SD age was 41.6±7.1 years, and their mean term of experience was 10.1±7.8 years. Forty (80 percent) were working with inpatients, five (10 percent) were working with outpatients, and five (10 percent) were working with both inpatients and outpatients.
Among the most frequently used sources of information (mean scores of 3 or greater) were journals, colleagues, morning rounds, congresses, textbooks, the Internet, and contacts with representatives of the pharmaceutical industry. The psychiatrists seldom used advertisements, the hospital's pharmacy, brochures from the pharmaceutical industry, or vocational training (mean score of less than 3).
All but one of the psychiatrists were aware of the new antipsychotic agent due to be introduced to the German market in three months. Ten (20 percent) of them had already had experiences with the drug from participating in clinical trials carried out for registration purposes. The first contact with the new product was at congresses (seven psychiatrists, or 14 percent) or through colleagues (five psychiatrists, or 10 percent), drug company representatives (13 psychiatrists, or 26 percent), the phase III study (14 psychiatrists, or 28 percent), journals (seven psychiatrists, or 14 percent), or in-house training (six psychiatrists, or 12 percent). Other forms of contact occurred through a patient (one psychiatrist, or 2 percent), the Internet (one psychiatrist, or 2 percent), or pharmacy (one psychiatrist, or 2 percent).
The psychiatrists were shown statements that documented the attributes of aripiprazole, which were derived from clinical trials. The statements are shown in Table 1 and were provided by the manufacturer of aripiprazole upon request. All attributes concerning efficacy and side effects were rated as being important for clinical practice ( Table 1 ). However, when asked how reliable these statements were, psychiatrists gave significantly lower ratings ( Table 1 ). The attributes related to the mechanism of action were rated as less important for clinical practice. Here no consistent difference between perceived importance and credibility was found.
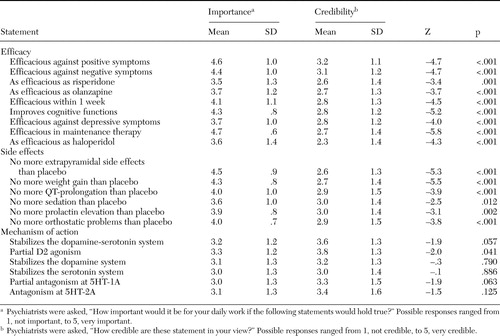 |
Forty-one psychiatrists (82 percent) planned to use the compound immediately upon market introduction.
A linear regression model that used psychiatrists' age, work experience, experience with aripiprazole within clinical trials, uncertainty tolerance, and number of voluntarily treated patients under supervision was applied to predict whether a psychiatrist attributed high or low credibility to the statements. Therefore, a sum score of all credibility items was derived for each category (efficacy, side effects, and mechanism of action) and served as a dependent variable. Cronbach's α was .89 for the statements on efficacy, .90 for those on side effects, and .90 for those on mechanism of action.
In regard to efficacy and side effects, the age and experience of the psychiatrists predicted the extent to which they believed the attributes presented would hold true; older psychiatrists (p=.02 for efficacy and p=.002 for side effects) and more experienced psychiatrists (p=.01 and p=.003, respectively) were more skeptical (R 2 =1 7 and 28 percent, respectively). No such relationship was found for the items on mechanism of action.
The patients for whom aripiprazole was considered suitable were younger (Mann-Whitney, Z=-2.59, p=.01), had a shorter duration of illness (Mann-Whitney, Z=-2.56, p=.01), and had fewer previous hospitalizations (Man-Whitney, Z=-2.93, p=.003). The patients not likely to have aripiprazole prescribed were more often prescribed first-generation antipsychotics (χ 2 =10.5, df=2, p=.005). No further differences were found between the two groups.
Three months after the introduction of aripiprazole, 37 of 47 psychiatrists had already prescribed the drug (79 percent).
A logistic regression model that used several variables—psychiatrist's age, work experience, experience with aripiprazole, uncertainty tolerance, and number of voluntarily treated patients under his or her supervision—was applied to predict whether a psychiatrist would adopt the new drug within the first three months. The model predicted 43 percent of the variance (R 2 =.43). Older psychiatrists (p=.033), those treating more patients on a voluntary basis (p=.022), and those expressing higher uncertainty tolerance (p=.045) were more likely to have prescribed aripiprazole within three months after its launching.
Discussion
No delay in the adoption of aripiprazole by the psychiatrists was found. Reasons for this finding might be because psychiatrists were well informed and because some of them had already had experiences with the drug before the market introduction. Most of them had their first contact with aripiprazole through phase III studies or drug company representatives. Thus, in the case of aripiprazole, the information on the drug had been disseminated very effectively. Hence aripiprazole might fall into that category of new drugs in which public demand boosts implementation ( 3 ).
It can be viewed as positive that psychiatrists were well informed before prescribing a new compound and were willing to adopt innovations at an early stage. However, the influence of the manufacturers might conceivably lead to biased information and unfavorable influence on psychiatrists' decision making. In the case of aripiprazole, the market introduction had been postponed several times because registration was delayed, with the marketing having already started before the launch.
Some skepticism on the psychiatrists' part was found in the discrepancy between the perceived importance of certain drug characteristics and the credibility of the description. Apparently psychiatrists had a desire for an effective drug without side effects but doubted whether aripiprazole would fulfill these expectations. Experiences with previous market introductions may have played an important role here. One might even suggest that the more market introductions a psychiatrist has witnessed, the more skeptical he or she becomes toward marketing strategies. However, this skepticism, adequately expressed in our interviews, had no influence on the psychiatrists' prescription of the new drug. On the contrary, psychiatrists who expressed the greatest concerns (the older psychiatrists) were more likely to be early adopters of the drug.
This finding not only appears paradoxical but also contradicts earlier findings of our research group that showed that older physicians were more likely to prescribe older drugs ( 12 ). One explanation for this discrepancy might be that in the study presented here, we interviewed only hospital psychiatrists (who are less autonomous in their habits), whereas in the previous study, we also studied psychiatrists in private practice. Not only did psychiatrists' age predict the early adoption of the drug, but their working environment and their personality characteristics also affected whether they adopted the drug within three months after launch. Thus psychiatrists who worked with patients undergoing treatment voluntarily were more likely to adopt the drug early. This finding goes hand in hand with the finding that the perceived "ideal" patient for aripiprazole tends to be mildly ill and under treatment with second-generation antipsychotics, whereas the patients with more critical illness (treated on an involuntary basis) might still be prescribed well-known, first-generation drugs. This behavior might reflect skepticism as to the efficacy of the new compound or a strategy of cautious action.
If the latter is the case, the dissemination of new drugs would be expected to begin with the patients with mild illness and proceed to those with more severe mental illness. This strategy is of interest, because an opposite one could also be imagined (treating nonresponders with a drug having a new mechanism of action).
From a review of these results it might be concluded that we have found a perfect example of rapid implementation of an innovative drug. However, neither we nor the psychiatrists studied know whether aripiprazole will really prove to be an effective and tolerable agent that is superior to its comparators (see the discussion of second-generation antipsychotics in studies by Rosenheck and colleagues [8] and Lieberman and colleagues [9]). This question can be answered only after intensive use of the agent under naturalistic conditions and examination in randomized controlled trials.
More important in our view, however, are the observed variations in the psychiatrists' prescribing behavior that are based on the psychiatrists' characteristics—age, working environment, or uncertainty tolerance. In view of the recent debates about whether second-generation antipsychotics really have advantages over first-generation antipsychotics or whether selective serotonin reuptake inhibitors are used too frequently, it seems vital to obtain a deeper insight into how psychiatrists adopt new drugs and how information or education about new therapies should best be disseminated.
Because this was an exploratory study, statistical statements are to be seen as generating rather than confirming hypotheses. It is at least possible that our presentation of information on aripiprazole during the first interviews might to some extent have worked as an intervention. On the other hand, it is a strength of the survey that it was undertaken prospectively (before the introduction of the compound) and that we conducted a follow-up survey with the same psychiatrists after the launch of the drug.
Conclusions
Hospital psychiatrists were shown to be early adopters of a new antipsychotic compound. This finding might be due to the fact that the psychiatrists were confronted even before market introduction with a wealth of information on the drug, mainly from the manufacturer. Despite their expressed skepticism about the drug's claims, most psychiatrists had no inhibitions about prescribing the new drug within three months after market introduction. It is not clear whether the psychiatrists adopted an important innovation at an early stage, thereby giving the best treatment available to their patients, or whether their prescribing practice was disadvantageously influenced by marketing strategies and a more cautious adoption of the new drug would be desirable. Finally, the impact of practice variation requires further evaluation.
Acknowledgments
The authors thank Gerd Laux, M.D., Herbert Pfeiffer, M.D., Max Schmau?, M.D., and Wolfgang Schreiber, M.D., for their support. The authors also thank Rudolf Cohen, Ph.D, and Claudia Dalbert, Ph.D., for their advice. The survey was funded in part by an unrestricted grant from Bristol-Myers Squibb, Germany.
1. Haines A, Jones R: Implementing findings of research. British Medical Journal 308:1488-1492, 1994Google Scholar
2. Solomon DH, Schneeweiss S, Glynn RJ, et al: Determinants of selective cyclooxygenase-2 inhibitor prescribing: are patient or physician characteristics more important? American Journal of Medicine 115:715-720, 2003Google Scholar
3. Steffensen FH, Sorensen HT, Olesen F: Diffusion of new drugs in Danish general practice. Family Practice 16:407-413, 1999Google Scholar
4. Tamblyn R, McLeod P, Hanley JA, et al: Physician and practice characteristics associated with the early utilization of new prescription drugs. Medical Care 41:895-908, 2003Google Scholar
5. Woodward ND, Purdon SE, Meltzer HY, et al: A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. International Journal of Neuropsychopharmacology 8:457-472, 2005Google Scholar
6. Leucht S, Pitschel-Walz G, Abraham D, et al: Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophrenia Research 35:51-68, 1999Google Scholar
7. Leucht S, Wahlbeck K, Hamann J, et al: New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet 361:1581-1589, 2003Google Scholar
8. Rosenheck R, Perlick D, Bingham S, et al: Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. JAMA 290:2693-2702, 2003Google Scholar
9. Lieberman JA, Stroup TS, McEvoy JP, et al: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209-1223, 2005Google Scholar
10. Galanter CA, Patel VL: Medical decision making: a selective review for child psychiatrists and psychologists. Journal of Child Psychology and Psychiatry 46:675-689, 2005Google Scholar
11. Dalbert C: Die Ungewissheitstoleranzskala: Skaleneigenschaften und Validierungsbefunde [The Uncertainty Tolerance Scale: Properties and Validation]. Halle, Germany, Martin-Luther-Universität Halle-Wittenberg, 1999Google Scholar
12. Hamann J, Langer B, Leucht S, et al: Medical decision making in antipsychotic drug choice for schizophrenia. American Journal of Psychiatry 161:1301-1304, 2004Google Scholar



