From Bedside to Bench: How the Epidemiology of Clinical Practice Can Inform the Secondary Prevention of PTSD
Each year in the United States 37 million acute care visits are made by patients in the wake of traumatic physical injuries; 2.5 million Americans are so severely injured that they require inpatient hospitalization ( 1 ). Trauma exposure, when coupled with physical injury, confers a higher risk for the development of posttraumatic stress disorder (PTSD) ( 2 , 3 , 4 , 5 ). Between 10 and 50 percent of injured youths and adults who are hospitalized develop high levels of PTSD symptoms ( 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ). As a high-risk group, injured trauma survivors have been targeted for early PTSD screening and intervention ( 15 , 16 ).
A diverse group of candidate compounds, including corticosteroids, beta-adrenergic antagonists, and opiate analgesics, have been theoretically articulated as early-intervention agents in the secondary psychopharmacologic prevention of PTSD ( 17 , 18 , 19 , 20 ). Among these potential agents, corticosteroids and beta-adrenergic antagonists have been selected for initial randomized trials in acute care settings ( 18 , 21 , 22 , 23 , 24 ). A literature review, however, revealed no published reports of early-intervention trials with analgesics.
Health services researchers have used pharmacoepidemiologic studies to expand the knowledge base regarding usual-care prescription patterns, off-label medication use, and assessments of medication safety and adverse events ( 25 , 26 , 27 , 28 ). Although many medications may be proposed as potential agents to prevent PTSD, some are used more than others in practice. Learning about these rates and patterns of use in real-world acute care practices will help in identifying which agents may be easier to test in randomized clinical trials. It is easier (and more likely to be more feasible in practice) to test a medication that is already in widespread use than one that may have too many contraindications in acute care settings. The testing of new compounds, however, is indicated only if there is also some theoretical basis for expecting that one of these agents might prevent PTSD. Although there are prior investigations of usual-care PTSD pharmacotherapy in outpatient veteran and civilian samples ( 29 , 30 ), few have been conducted in acute care settings.
The goal of this investigation was to use clinical epidemiologic methods to inform pharmacological intervention development in the secondary prevention of PTSD. We used clinical epidemiologic methods in an effort to attain a representative sample of injured adolescents and adults admitted to a level 1 trauma center. We hypothesized that cross-sectional data on routine use of acute care medication would reveal high frequencies of analgesic prescription at the time of hospital discharge, consistent with high rates of self-reported physical health and bodily pain concerns immediately after an injury ( 31 ). We were also interested in documenting the frequencies of usual administration of other classes of medication (corticosteroids and beta-adrenergic blockers) that are currently being tested as candidate compounds for secondary prevention of PTSD.
Methods
Data for the study were derived from previous investigations of injured youths and adults. Details of investigative methods have been published elsewhere ( 7 , 11 ). Below we highlight specific procedures of relevance to this report.
Participants
Adolescent (ages 12 to 18) and adult (ages 18 and older) survivors of intentional injuries (those associated with human malice, such as physical assaults), and unintentional injuries (such as motor vehicle crashes and injuries sustained on the job) were recruited from the University of Washington's Harborview Medical Center. Harborview is the only level 1 trauma center serving the states of Washington, Idaho, Montana, and Alaska and admits more than 6,000 injured trauma survivors of all ages each year. Patients with severe injuries that prevented study participation (such as severe head or spinal cord injuries), patients found to have self-inflicted injuries, and non-English-speaking patients were excluded from the study.
All informed consent procedures and automated medical record analyses were approved by the University of Washington Institutional Review Board. Full informed consent was obtained from all patients before data collection. For patients under the age of 18, adolescent assent and parental consent were obtained.
Each weekday morning a research associate downloaded a list of all newly admitted injury survivors from the Harborview automated admissions database. Microsoft Excel spreadsheet software was used to generate random number assignments for each newly admitted patient. The research associate then approached each potential participant in the surgical ward in the order dictated by the random number assignments.
Data analyses
To assess the representativeness of the sample, we first compared the demographic and injury characteristics of patients included in the investigation with the characteristics of all patients admitted to Harborview trauma surgery services during the period of study recruitment. Next, medication prescription data contained within each patient's electronic medical record were downloaded by the study research associate. The first author assigned each prescribed medication to an appropriate class on the basis of categories described in the Harborview Hospital medication formulary. We tabulated, by medication category, the frequencies of medication prescribed at hospital discharge for adult and adolescent patients. Of note, medications prescribed only on an as-needed basis (such as aluminum hydroxide or magnesium hydroxide as needed for dyspepsia) were not included in the analyses.
Results
Injured adolescents were recruited from Harborview between July 2002 and August 2003. The 113 adolescent inpatients included in the study did not significantly differ from the total of 561 injured adolescents admitted to Harborview trauma surgical services with regard to gender, age, injury type, and injury severity. With regard to ethnocultural heritage, 86 adolescents (76 percent) were white, six (5 percent) were Hispanic, six (5 percent) were African American, three (3 percent) were Asian, three (3 percent) were American Indian, and nine (8 percent) were from mixed or other backgrounds. The mean±SD length of inpatient stay for the 113 adolescent patients was 5.3±6.3 days; 28 adolescents (25 percent) were admitted to the intensive care unit, and the median length of stay in the intensive care unit was one day (range of one to 15 days).
Between March 2001 and January 2002, we recruited 152 injured adults, who did not significantly differ from all other adult patients admitted (N=2,358) to Harborview trauma surgical services with regard to gender, intentional injury, and injury severity. Adult patients included in the investigation were on average younger than patients not included in the investigation (mean=38±14.7 years versus 42±18.3 years; t=2.7, df=2,509, p= .03). Adult patients were from diverse ethnocultural backgrounds: 96 (63 percent) were white, 20 (13 percent) were African American, 15 (10 percent) were Hispanic, 13 (9 percent) were American Indian, and eight (5 percent) were Asian. The average length of stay for the 152 adult inpatients was 5.9±5.6 days. Nineteen adults (13 percent) were admitted to the intensive care unit, and the median length of stay in the intensive care unit was two days (range of one to seven days).
At hospital discharge, more than 80 percent of patients received opiate analgesics (such as oxycodone), and opiate analgesics were therefore the most commonly prescribed medication class ( Table 1 ). Nonopiate analgesics (such as ibuprofen) were prescribed to 51 (45 percent) adolescents and 52 (34 percent) adults. Benzodiazepines (such as lorazepam), anticonvulsants (such as phenytoin), beta-adrenergic blockers (such as propranolol), corticosteroids (such as prednisone), and gabapentin were prescribed to less than 10 percent of the patients ( Table 1 ).
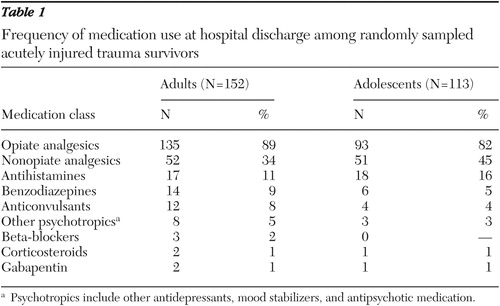 |
A final category of other psychotropic medications was created for antidepressant, antipsychotic, or other psychopharmacological agents that were not in the previously identified medication classes. Psychopharmacological agents prescribed included risperidone for three patients; trazodone for two patients; venlafaxine for two patients; and methylphenidate, mirtazapine, naloxone, olanzapine, and sertraline, each for one patient.
Discussion
This investigation identified usual-care prescription patterns in a representative sample of traumatically injured adolescent and adult acute care inpatients. The investigation documented that opiate analgesics were prescribed for more than 80 percent of the patients, whereas approximately one-third of patients were receiving nonopiate analgesic prescriptions at the time of hospital discharge. Benzodiazepines, corticosteroids, beta-adrenergic blockers, and other psychotropic medications were prescribed to less than 10 percent of patients.
This investigation has potential implications for the development of acute care pharmacotherapeutic interventions targeting the secondary prevention of PTSD. Development of pharmacological interventions has been conceptualized as a unidirectional progression from drug development, through efficacy studies, and then effectiveness trials ( 32 , 33 ). Previous commentary has emphasized the importance of effectiveness trials with representative patient samples to ensure the development of medication interventions that may be robustly applied to real-world treatment settings ( 34 , 35 , 36 , 37 ). Other commentary suggests that methodologically diverse investigations that cut across efficacy, effectiveness, and dissemination paradigms may optimally inform intervention development ( 26 , 38 , 39 , 40 , 41 , 42 , 43 ). Taken together, the findings of this study and these commentaries highlight the potential for practice research to respond and inform stages of intervention development, such as treatments selected for inclusion in efficacy trials ( 40 ).
The investigation identified markedly different acute care prescribing patterns for compounds with strong theoretical rationales for the prevention of PTSD. For example, few surgical inpatients were receiving corticosteroids. Although corticosteroids are safe in highly monitored intensive care unit settings ( 22 , 23 ) where previous trials have been done, they may be contraindicated in the surgical inpatient ward setting where they place patients with penetrating abdominal injuries at risk for both intestinal perforation and sepsis ( 44 ). Similarly, beta-blockers may be contraindicated for acute care inpatients with hypovolemia and associated cardiovascular instability ( 45 ). Beta-adrenergic antagonists may be more appropriate for injury survivors who are evaluated in emergency department settings but not admitted to the hospital ( 21 , 46 ).
Our investigation documented that analgesic medications are widely prescribed in the acute care setting at the time of inpatient hospital discharge, consistent with the predominance of pain complaints over psychological concerns in the days and weeks immediately after injury ( 31 ). Opiates have been identified as potent anxiolytics in animal models ( 47 ), and pain responses appear to be regulated in part by centrally mediated catecholamine metabolism ( 48 , 49 ). Also, opiates may prevent memory consolidation through a beta-adrenergic mechanism ( 50 ). Adequate levels of opiate pain control are associated with the development of lower PTSD symptom levels among burn injury survivors who are children ( 20 ). Thus opiate analgesics may be important agents to consider for future acute care efficacy trials targeting PTSD prevention ( 17 ).
The extensive use of narcotic analgesics may be contraindicated for subpopulations of acute care patients; between 20 and 40 percent of acute care inpatients have been given diagnoses (current or lifetime) of substance abuse or dependence ( 51 , 52 , 53 ). Thus newer agents such as gabapentin and pregabalin, with combined analgesic and anxiolytic properties, may hold promise as early preventive agents for testing in efficacy trials ( 54 , 55 , 56 , 57 ). Also, basic research might focus on the development and testing of novel compounds with combined analgesic and anxiolytic properties. Future prospective studies in acute care settings could assess the prevalence of PTSD among patients taking medications with a hypothesized preventive effect at the time of admission for injury.
There are important considerations in interpreting data from the investigation. The finding that corticosteroids and beta-adrenergic agents are infrequently prescribed at the time of hospital discharge may reflect that there is no current indication for these medications rather than adverse side effect profiles. Also, epidemiologic studies of commonly prescribed nonopiate analgesics suggest multiple indications beyond pain control (such as prevention of adverse cardiac outcomes). Therefore, the investigation may overestimate prescription of nonopiate analgesic medication at the time of hospital discharge for targeting pain control ( 58 ). Also, the investigation was conducted at a single level 1 trauma center. Representative sampling frameworks could be used to confirm similar prescription patterns for the 1,154 trauma centers nationwide ( 59 ). Finally, we note that the pharmacoepidemiologic method we used in the investigation is useful only when preexisting theoretical rationales exist for multiple compounds. We do not advocate the testing of widely used compounds for new indications without preexisting rationales.
Conclusions
A major challenge facing psychiatric research is the development of interventions that have both a theoretical rationale for affecting the pathophysiology of the disorder in question and the ability to be robustly applied across diverse real-world settings ( 40 ). The results of this investigation substantiate the ubiquitous use of analgesic medication in the acute care inpatient setting. These data, and preclinical evidence indicating an overlap between pain and anxiety pathways, and analgesia and anxiolysis, suggest initial feasibility tests and efficacy trials of compounds targeting pain in the secondary prevention of PTSD after injury ( 60 ). Basic research could also be conducted on compounds that simultaneously target pain and anxiety. The investigation demonstrates how population-based data derived from real-world practice settings can enhance the efficiency (such as selection of compounds more robustly applied to real-world treatment settings) and trajectories (such as basic research on promising compounds) of pharmaceutical intervention development.
Acknowledgments
This work was supported in part by grant MH-01610 from the National Institute of Mental Health and grant HS-11372 from the Agency for Healthcare Research and Quality.
1. Bonnie RJ, Fulco CE, Liverman CT: Reducing the Burden of Injury: Advancing Prevention and Treatment. Washington, DC, National Academy Press, 1999Google Scholar
2. Hoge CW, Castro CA, Messer SC, et al: Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine 351: 13-22, 2004Google Scholar
3. Helzer JE, Robins LN, McEvoy L: Post-traumatic stress disorder in the general population: findings of the Epidemiological Catchment Area survey. New England Journal of Medicine 317:1630-1634, 1987Google Scholar
4. Green BL: Identifying survivors at risk, in International Handbook of Traumatic Stress Syndromes. Edited by Wilson JP, Raphael B. New York, Plenum, 1993Google Scholar
5. Koren D, Norman D, Cohen A, et al: Increased PTSD risk with combat-related injury: a matched comparison study of injured and uninjured soldiers experiencing the same combat events. American Journal of Psychiatry 162:276-282, 2005Google Scholar
6. Michaels AJ, Michaels CE, Moon CH, et al: Psychosocial factors limit outcomes after trauma. Journal of Trauma 44:644-648, 1998Google Scholar
7. Zatzick DF, Kang SM, Muller HG, et al: Predicting posttraumatic distress in hospitalized trauma survivors with acute injuries. American Journal of Psychiatry 159:941-946, 2002Google Scholar
8. Ursano RJ, Fullerton CS, Epstein RS, et al: Acute and chronic posttraumatic stress disorder in motor vehicle accident victims. American Journal of Psychiatry 156:589-595, 1999Google Scholar
9. Mellman TA, David D, Bustamante V, et al: Predictors of post-traumatic stress disorder following severe injury. Depression and Anxiety 14:226-231, 2001Google Scholar
10. Kassam-Adams N, Winston FK: Predicting child PTSD: the relationship between acute stress disorder and PTSD in injured children. Journal of the American Academy of Child and Adolescent Psychiatry 43:403-411, 2004Google Scholar
11. Zatzick DF, Russo J, Grossman D, et al: PTSD and depressive symptoms, alcohol use, and recurrent traumatic life events in a representative sample of injured adolescents and their parents. Journal of Pediatric Psychology 31:377-387, 2006Google Scholar
12. O'Donnell ML, Creamer M, Pattison P: Posttraumatic stress disorder and depression following trauma: understanding comorbidity. American Journal of Psychiatry 161:1390-1396, 2004Google Scholar
13. Shalev AY, Freedman S, Peri T, et al: Predicting PTSD in trauma survivors: prospective evaluation of self-report and clinician-administered instruments. British Journal of Psychiatry 174:558-564, 1999Google Scholar
14. Verger P, Dab W, Lamping DL, et al: The psychological impact of terrorism: an epidemiologic study of posttraumatic stress disorder and associated factors in victims of the 1995-1996 bombings in France. American Journal of Psychiatry 161:1384-1389, 2004Google Scholar
15. Mental Health and Mass Violence: Evidence-Based Early Psychological Intervention for Victims/Survivors of Mass Violence: A Workshop to Reach Consensus on Best Practices. NIH pub no 02-5138. Bethesda, Md, National Institute of Mental Health, 2002Google Scholar
16. Mental Health All-Hazards Disaster Planning Guidance. Rockville, Md, Center for Mental Health Services, Substance Abuse and Mental Health Services Administration, 2003Google Scholar
17. Friedman MJ: Future pharmocotherapy for post-traumatic stress disorder: prevention and treatment. Psychiatric Clinics of North America 25:427-441, 2002Google Scholar
18. Pitman RK, Delahanty DL: Conceptually driven pharmacologic approaches to acute trauma. CNS Spectrums 10:99-106, 2005Google Scholar
19. Schoenfeld FB, Marmar CR, Neylan TC: Current concepts in pharmacotherapy for posttraumatic stress disorder. Psychiatric Services 55:519-531, 2004Google Scholar
20. Saxe G, Stoddard F, Courtney D, et al: Relationship between acute morphine and the course of PTSD in children with burns. Journal of the American Academy of Child and Adolescent Psychiatry 40:915-921, 2001Google Scholar
21. Pitman RK, Sanders KM, Zusman RM, et al: Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological Psychiatry 51:189-192, 2002Google Scholar
22. Schelling G, Briegel J, Roozendaal B, et al: The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biological Psychiatry 50:978-985, 2001Google Scholar
23. Schelling G, Kilger E, Roozendaal B, et al: Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biological Psychiatry 55:627-633, 2004Google Scholar
24. Vaiva G, Ducrocq F, Jezequel K, et al: Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biological Psychiatry 54:947-949, 2003Google Scholar
25. Stein BD, Sturm R, Kapur K, et al: Psychotropic medication costs among youth with private insurance in 1998. Psychiatric Services 52:152, 2001Google Scholar
26. Zito JM, Safer DJ: Services and prevention: pharmacoepidemiology of antidepressant use. Biological Psychiatry 49:1121-1127, 2001Google Scholar
27. Bussing R, Zima BT, Mason D, et al: Use and persistence of pharmacotherapy for elementary school students with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 15:78-87, 2005Google Scholar
28. Raghavan R, Zima BT, Andersen RM, et al: Psychotropic medication use in a national probability sample of children in the child welfare system. Journal of Child and Adolescent Psychopharmacology 15:97-106, 2005Google Scholar
29. Rosen CS, Chow HC, Finney JF, et al: VA practice patterns and practice guidelines for treating posttraumatic stress disorder. Journal of Traumatic Stress 17:213-222, 2004Google Scholar
30. Mellman TA, Clark RE, Peacock WJ: Prescribing patterns for patients with posttraumatic stress disorder. Psychiatric Services 54:1618-1621, 2003Google Scholar
31. Zatzick DF, Kang SM, Hinton WL, et al: Posttraumatic concerns: a patient-centered approach to outcome assessment after traumatic physical injury. Medical Care 39:327-339, 2001Google Scholar
32. Weisz JR, Chu BC, Polo AJ: Treatment dissemination and evidence-based practice: strengthening intervention through clinician-researcher collaboration. Clinical Psychology: Science and Practice 11:300-307, 2004Google Scholar
33. Greenwald P, Cullen JW: The scientific approach to cancer control. CA: A Cancer Journal for Clinicians 34:328-332, 1984Google Scholar
34. Lagomasino IT, Dwight-Johnson M, Simpson GM: Psychopharmacology: the need for effectiveness trials to inform evidence-based psychiatric practice. Psychiatric Services 56:649-651, 2005Google Scholar
35. Roy-Byrne PP, Sherbourne C, Craske M, et al: Moving treatment research from clinical trials to the real world. Psychiatric Services 53:327-332, 2003Google Scholar
36. Duan N, Braslow JT, Weisz JR, et al: Fidelity, adherence, and robustness of interventions. Psychiatric Services 52:413, 2001Google Scholar
37. Sullivan G, Duan N, Mukherjee S, et al: The role of services researchers in facilitating intervention research. Psychiatric Services 56:537-542, 2005Google Scholar
38. Zatzick DF, Simon G, Wagner A: Developing and implementing randomized effectiveness trials to assess the delivery of efficatious mental health interventions in general medical settings. Clinical Psychology: Science and Practice 13:53-68, 2006Google Scholar
39. Schoenwald SK, Hoagwood K: Effectiveness, transportability, and dissemination of interventions: what matters when? Psychiatric Services 52:1190-1197, 2001Google Scholar
40. National Institute of Mental Health: Bridging Science and Service: A Report by the National Advisory Mental Health Council's Clinical Treatment and Services Research Workgroup. Bethesda, Md, National Institutes of Health, 1999Google Scholar
41. Kazdin A: Progression of therapy research and clinical application of treatment require better understanding of the change process. Clinical Psychology: Science and Practice 8:143-151, 2001Google Scholar
42. Rounsaville B, Carroll KM, Onken LS: Methodological diversity and theory in the stage model: reply to Kazdin. Clinical Psychology: Science and Practice 8:152-154, 2001Google Scholar
43. Holder H, Flay B, Howard J, et al: Phases of alcohol problem prevention research. Alcoholism, Clinical and Experimental Research 23:183-194, 1999Google Scholar
44. Kelly KA, Sarr MG, Hinder RA: Mayo Clinic Gastrointestinal Surgery. Philadelphia, Saunders, 2004Google Scholar
45. Hurst JM: Common Problems in Trauma. Chicago, Year Book Medical, 1987Google Scholar
46. Roy-Byrne PP, Berliner L, Russo J, et al: Treatment preferences and determinants in victims of sexual and physical assault. Journal of Nervous and Mental Disease 191:161-165, 2003Google Scholar
47. Golub MS, Kaaekuahiwi MA: Response to maternal separation in infant guinea pigs exposed to intrapartum meperidine. Developmental Psychobiology 28:59-68, 1995Google Scholar
48. Zubieta JK, Heitzeg MM, Smith YR, et al: COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 299:1240-1243, 2003Google Scholar
49. Vastag B: Scientists find connections in the brain between physical and emotional pain. JAMA 290:2389-2390, 2003Google Scholar
50. McGaugh JL, Introini-Collison IB, Nagahara AH: Memory-enhancing effects of posttraining naloxone: involvement of beta-noradrenergic influences in the amygdaloid complex. Brain Research 446:37-49, 1988Google Scholar
51. Soderstrom CA, Smith GS, Dischinger PC, et al: Psychoactive substance use disorders among seriously injured trauma center patients. JAMA 277:1769-1774, 1997Google Scholar
52. Zatzick DF, Jurkovich G, Russo J, et al: Posttraumatic distress, alcohol disorders, and recurrent trauma across level 1 trauma centers. Journal of Trauma: Injury, Infection, and Critical Care 57:360-366, 2004Google Scholar
53. Schermer CR, Wisner DH: Methamphetamine use in trauma patients: a population-based study. Journal of the American College of Surgeons 189:442-449, 1999Google Scholar
54. Sabatowski R, Galvez R, Cherry DA, et al: Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 109:26-35, 2004Google Scholar
55. Nemeroff CB: The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacology Bulletin 37:133-146, 2003Google Scholar
56. Spira PJ, Beran RG: Gabapentin in the prophylaxis of chronic daily headache: a randomized, placebo-controlled study. Neurology 61:1753-1759, 2003Google Scholar
57. Feltner DE, Crockatt JG, Dubovsky SJ, et al: A randomized, double-blind, placebo-controlled, fixed-dose, multicenter study of pregabalin in patients with generalized anxiety disorder. Journal of Clinical Psychopharmacology 23:240-249, 2003Google Scholar
58. Signorello LB, McLaughlin JK, Lipworth L, et al: Confounding by indication in epidemiologic studies of commonly used analgesics. American Journal of Therapeutics 9:199-205, 2002Google Scholar
59. MacKenzie EJ, Hoyt DB, Sacra JC, et al: National inventory of hospital trauma centers. JAMA 289:1515-1522, 2003Google Scholar
60. Zatzick DF, Roy-Byrne P: Developing high-quality interventions for posttraumatic stress disorder in the acute care medical setting. Seminars in Clinical Neuropsychiatry 8:158-167, 2003Google Scholar



