Characteristics of Subgroups of Individuals With Psychotic Illness and a Comorbid Substance Use Disorder
Abstract
OBJECTIVES: The co-occurrence of severe mental illness and substance use disorder, or dual diagnosis, is prevalent and is associated with significant clinical and social problems. Most studies have treated persons with a dual diagnosis as a homogeneous population, grouping together different substances of misuse. This study investigated whether subgroups defined by their main substances of misuse were heterogeneous. The primary hypothesis was that users of stimulants, such as cocaine or amphetamines, would be characterized by especially high rates of inpatient admission, violence, and self-harm. METHODS: Case managers' ratings were used to identify individuals with serious mental illness and comorbid substance abuse or dependence who were being treated by 13 community mental health teams in South London. Standardized instruments were used to elicit sociodemographic, clinical, social, and service use data. RESULTS: A total of 233 cases of comorbid substance use disorder and psychotic illness were identified. On the basis of best available information, 78 (34 percent) patients were classified as alcohol misusers only, 52 (22 percent) as alcohol and cannabis users, 29 (12 percent) as users of cannabis only, and 55 (24 percent) as stimulant users; 19 patients (8 percent) were excluded from the analysis. No significant differences were found between subgroups in the use of inpatient services and lifetime history of self-harm, but there was a significant difference in lifetime history of violence, which was more frequent among stimulant users. The alcohol users were older and more likely to be white, but otherwise few differences between subgroups were suggested by exploratory analyses. CONCLUSIONS: Apart from differences in history of violence, little heterogeneity was found among subgroups of patients with different types of substance misuse.
Substance misuse among persons with psychotic illness has caused increasing concern on both sides of the Atlantic. Reported adverse outcomes in this patient population include hospitalization, noncompliance with medications, suicide, violence, homelessness, and HIV infection (1). The estimated prevalence of comorbid substance misuse varies between 20 percent and 60 percent of persons who have a diagnosis of a severe mental illness in the United States (2,3,4,5,6), the United Kingdom (7,8,9,10,11,12,13,14), and Europe (15,16,17,18,19,20,21). An important question for understanding the etiology of co-occurring substance misuse and severe mental illness and for needs assessment and service planning is whether there are subgroups of this population with distinct characteristics and patterns of need (22,23), given that it has been said that "the typical dual diagnosis patient is a mythical creature" (22).
Some authors have proposed subgroups based on psychiatric diagnosis or the temporal relationship between the onset of substance misuse and of mental illness (24,25,26). Relationships have also been reported between patient characteristics and sociodemographic variables such as gender, ethnicity, and whether the person lives in an urban or a rural setting (4,27,28,29,30,31). However, detailed investigation of whether differences exist in characteristics, needs, and service use between groups of persons who use different substances has remained rare, with many authors treating persons with any form of substance misuse as a homogeneous category (32).
The legitimacy of grouping together alcohol and drug misusers or those who use only cannabis with misusers of multiple substances is uncertain, particularly given that the psychopharmacologic effects and social contexts of use of the different substances vary considerably. A contrasting approach is often taken in research and service development relating to people who have substance use disorders only, where the focus is often specifically on alcohol or on individual drugs rather than the whole spectrum of substances of abuse.
In this study, we investigated whether substance of choice is a useful basis for distinguishing subgroups of persons with a dual diagnosis. We focused exclusively on persons with both a primary psychotic illness and comorbid substance misuse, who themselves constitute an important clinical subgroup among persons who have both substance use problems and mental health problems. Our aims were to describe patterns of substance use in a sample from South London and to investigate whether there were differences between subgroups of persons who misused alcohol only, alcohol and cannabis, cannabis only, and stimulants (alone or in addition to alcohol and cannabis).
The finding of Mueser and colleagues (29) of an association between cocaine use and criminal involvement and homelessness among persons with a dual diagnosis is one of few reports of variations between severely mentally ill users of different substances. On the basis of this finding and research on stimulant misusers who do not have mental illness (33), our primary hypothesis was that because of effects of stimulants on symptoms, the lifestyle associated with stimulant use, or use of multiple substances—which is frequent among stimulant users—a higher prevalence of history of self-harm and violence and greater use of inpatient beds would be found among those who used stimulants than among those who used other substances. A secondary hypothesis was that there would be differences between substance-defined subgroups in psychiatric and physical symptoms, compliance with medications, service satisfaction, level of need, and social functioning, again indicating more severe difficulties in the subgroup of stimulant users.
Methods
Data used in this cross-sectional study were collected at the baseline stage of the COMO randomized controlled trial, an evaluation of the effects of a training intervention on case managers' knowledge and attitudes about dual diagnosis and on clinical and social outcomes of their clients. After baseline data collection, case managers were randomly allocated to either an experimental group who received training immediately or a control group who received training after 18 months. Thirteen community mental health teams participated. One of these teams is a specialist team for the homeless mentally ill population of South London, and each of the others is responsible for all severely mentally ill individuals who live in the geographic catchment area served by the team.
Throughout the United Kingdom, the private sector provides little care for people with severe mental illness, with the result that the caseloads of community mental health teams are highly representative of local populations of persons with severe mental illness who are receiving mental heath care. Case managers participated in the study unless they were temporary staff or had firm plans to leave their jobs during the next 18 months. Allocation of new cases within teams is usually determined by available space on caseloads rather than by the special interests of team members, so the caseloads of case managers who did not participate in the study were likely to be similar to those of case managers who did participate, although we did not test this assumption empirically.
Approval for the study was obtained from the relevant local research ethics committees. Data collection took place in two phases between November 1999 and September 2000.
Phase 1: screening
Clients with an ICD-10 diagnosis (made by psychiatrists and recorded in case notes) of schizophrenia (code F20) or schizoaffective disorder (F295), bipolar affective disorder (F296), or delusional disorder (F297) who were on the caseloads of participating case managers were identified. After receiving individual guidance about the study instruments and rating categories from the researchers, the case managers rated each of these clients by using the Clinician Alcohol Use Scale (CAUS) and the Clinician Drug Use Scale (CDUS) (3). These scales provide operational definitions for classifying clients' use of alcohol and drugs in the following categories: abstinence, use without impairment, abuse, dependence, and dependence with institutionalization. Clients who were rated as abusing or dependent on at least one substance met the study criteria for dual diagnosis.
Phase 2: detailed assessment of clients
The case managers completed the following measures for all clients who met the study criteria for dual diagnosis in phase 1: the Medication Compliance Scale (34), the Camberwell Assessment of Need Short Assessment Schedule (CANSAS) (35), and the Life Skills Profile (LSP) (36), which assesses social functioning. The researchers interviewed patients from whom written informed consent was obtained and assessed service satisfaction by using the Treatment Perception Questionnaire (TPQ) (37) and the Client Satisfaction Questionnaire (CSQ) (38), drug and alcohol consumption by using the Alcohol Use Disorders Identification Test (AUDIT) (39) and the Maudsley Addiction Profile (MAP) (40), physical symptoms by using a section of the MAP that asks how frequently ten common physical symptoms have been experienced in the previous 30 days, and psychiatric symptoms by using the Expanded Brief Psychiatric Rating Scale (41). Sociodemographic characteristics, lifetime history of self-harm and violence (Clinical and Social History Scale [CSHS], designed specifically for COMO), and service use data (Client Service Receipt Inventory [42]) were recorded for all clients on the basis of the best available information obtainable from patients, staff, and case notes.
Classification of substance use
Substances of abuse were grouped so as to be meaningful in terms of common presentations to services (10,14,24). For the main analyses, the patients were classified into substance-of-choice subgroups on the basis of the best available information from all sources. Patients who were interviewed and acknowledged substance use were categorized on the basis of their own reports. In the case of two patients who were interviewed and denied any substance use and all patients whom we were unable to interview, we assumed that the next best source was their case managers' assessment of whether they abused alcohol or used illicit drugs, and they were classified according to case managers' ratings on the CAUS and the CDUS (3).
Patients were defined as misusing alcohol if they scored above 8, the cutoff point indicating problematic drinking on the AUDIT, or were drinking more than the U.K. weekly recommended limits: 21 units of alcohol for men and 14 units for women (43).
For other substances, any reported use in the previous month was considered potentially problematic and was used as a basis for categorization; the observation that persons with severe mental illness may be vulnerable to adverse effects from even low levels of drug use informed the choice of this low threshold (4,32). In categorizing the alcohol and cannabis subgroup, the above criteria for abuse for both substances had to be met. As a sensitivity analysis, we also investigated the effects of different ways of categorizing substance of choice. The main analyses were repeated first with subgroup classification based on case managers' ratings only for the whole sample and then based on patient interview data only, with only those interviewed included.
Analysis
Initial box plots of the distribution of continuous variables indicated that data on days participants spent as an inpatient were skewed, so they were analyzed by using the Kruskal-Wallis nonparametric test. For all other continuously distributed variables, analysis of variance was used to compare subgroups. For categorical data, subgroups were compared by using chi square tests or Fisher's exact tests as appropriate.
Except for the primary hypotheses relating to self-harm, violence, and bed use, p values were adjusted by using the Bonferroni correction. Where global F tests for analysis of variances yielded statistically significant results (after Bonferroni correction as appropriate), post hoc Bonferroni contrasts were used to compare pairs of subgroups. Standardized residuals were used to indicate sources of variation where global results from chi square tests were statistically significant. In the case of the only primary null hypothesis that resulted in significant rejection on univariate analysis, logistic regression was used to assess the effect of potential confounders.
Results
Seventy-eight case managers participated in the study. Of the 1,560 clients on the caseloads of these case managers, 1,271 had a clinical diagnosis of psychotic illness, of whom 233 met our criteria for dual diagnosis by being rated as abuse, dependence, or dependence with institutionalization on the CAUS or the CDUS and were included in the analysis. A total of 160 patients (69 percent) agreed to be interviewed; the remaining 73 refused, were too ill, or could not be located. No significant differences were found between participants and nonparticipants in psychiatric diagnosis, sex, ethnicity, or marital status, but differences in age were noted—mean±SD age of 39±11.1 years for participants and 35±9.4 for nonparticipants (t=2.2, df=231, p=.032)—and living situation (χ2=14.1, df=3, p=.003), with homeless persons being less likely to be interviewed.
Substance use and subgroup classification
Self-reported substance use in our sample is consistent with the results of previous research in the United Kingdom (10,11,12,13,14). Of the patients interviewed, 128 (80 percent) had drunk alcohol in the previous month. The mean weekly consumption among those who drank was 55.2±104.2 units for men and 22±24 units for women, with 50 percent of men (N=53) and 42 percent of women (N=10) drinking more than recommended U.K. limits (43). The mean AUDIT score was 12.3±9.7 (possible scores range from 0 to 40); 58 percent of the patients (N=64) attained scores above the instrument's problematic drinking threshold of 8.
Fifty-eight (64 percent) of those interviewed had smoked cannabis in the previous month. The mean number of cannabis-using days was 11.5±11.7, with 13 cannabis users (23 percent) using the substance daily. Twenty-five of the patients interviewed (16 percent) reported use of cocaine or crack cocaine in the previous 30 days. Of these, 19 had smoked, one had injected, and one had both injected and smoked crack, and four had snorted cocaine. The mean number of cocaine-using days in the previous month was 10.1±9.6, and average drug expenditure on a typical using day was 54 pounds sterling ($85). Other stimulants used were amphetamines (three participants), khat (three participants), and ketamine (one participant). Eight participants (5 percent) reported use of opiate drugs in the previous month, another three had used ecstasy, two solvents, two illicit benzodiazepines, and one cough linctus. Six of those interviewed (4 percent) had used drugs intravenously, including two patients who had shared needles. However, the most prevalent substance of misuse was tobacco, which was smoked by 150 (94 percent) of the patients interviewed.
Of the patients interviewed, 58 (36 percent) met our criteria for misuse of alcohol only, 21 (13 percent) for use of cannabis only, and 32 (20 percent) for both misuse of alcohol and use of cannabis. An additional 36 patients (23 percent) reported stimulant use and were assigned to the stimulants subgroup. Finally, 13 patients (8 percent) were excluded from further analyses because this "other" group was neither sufficiently large nor homogeneous for meaningful statistical analysis.
The 73 patients who were not interviewed were categorized on the basis of case managers' ratings only for the main analyses. Comparing patient and case manager reports, 46 (82 percent) of those assigned on the basis of patient reports to the alcohol-only group, 17 (55 percent) of those in the alcohol and cannabis group, 15 (71 percent) of the cannabis-only group, 24 (62 percent) of the stimulants-only group, and ten (77 percent) of the "other" group remained in the same category when case manager ratings rather than patients' self-reports were the basis of classification. No significant differences were observed between patients who were interviewed and those who were not in the proportions assigned to each subgroup.
After the interviewed and noninterviewed patients were combined, 78 of 233 clients (33 percent) were classified into the alcohol-only subgroup, 52 (22 percent) the alcohol-and-cannabis subgroup, 29 (1 percent) the cannabis-only subgroup, and 55 (24 percent) the stimulants subgroup. The remaining 19 (8 percent) were in the "other" subgroup and were excluded from further analyses. Thus 214 patients were included in the main analysis.
Sociodemographic differences between subgroups
The sociodemographic characteristics of the subgroups are summarized in Table 1. The only significant demographic differences between the groups, after Bonferroni correction, were in age and ethnicity. Post hoc Bonferroni contrasts indicated that clients in the alcohol-only subgroup were older than those in the other groups, which is consistent with the results of previous research (44). Examination of the standardized residuals indicated that patients in the alcohol-only subgroup were more likely to be white European, whereas those in the cannabis-only subgroup were more likely to be black Caribbean, black African, or black British. Differences in age and ethnicity were still significant when the analyses were repeated first with the subgroups based on the case managers' ratings and then with the subgroups based on patients' self-reported substance use.
Primary analysis. The primary analysis assessed differences in the use of inpatient services and lifetime history of self-harm or violence. The results are summarized in Table 2. No clearly significant differences in service use or self-harm emerged between subgroups. However, a significant difference in lifetime history of violence was noted. Examination of the standardized residuals indicated, as we hypothesized, that the stimulant users were significantly more likely to have ever committed a violent act. Logistic regression in which violence was the dependent variable and stimulant use was an explanatory variable alongside age, sex, marital status, and ethnicity was used to assess the effects of potential confounders. The significant association between violence and substance use persisted with this adjustment.
As a sensitivity analysis, the analyses described above were repeated with the subgroups categorized on the basis of case managers' ratings. The significant difference in violence between subgroups persisted (χ2= 11.5, df=3, p=.009). However, when the subgroups were based on the patients' self-reports only, the findings, although similar, were not statistically significant, possibly because the samples were small—violence was reported for 17 patients (30 percent) in the alcohol-only subgroup, seven (23 percent) in the alcohol-and-cannabis subgroup, five (24 percent) in the cannabis-only subgroup, and 17 (45 percent) in the stimulants subgroup.
Secondary analyses. The secondary analyses assessed differences in physical and psychiatric symptoms, compliance with medications, service satisfaction, level of need, and social functioning between subgroups. The results are summarized in Table 3.
On most indicators, little evidence emerged of differences between subgroups. Differences in service satisfaction (measured by the TPQ) and the social contact subscore of the LSP were not significant after Bonferroni correction. However, a difference in self-care scores persisted. Post hoc Bonferroni contrasts indicated that the alcohol-only subgroup had significantly poorer self-care (p<.01). The analyses were repeated, as before, to compare subgroups based on either case managers' or patients' categorizations. The findings were the same as for the combined categorizations except that a difference in social contact persisted after Bonferroni correction for subgroups based on patients' categorizations. The cannabis-only subgroup had the poorest social contact (F=6.3, df=3, 157, p<.001).
Discussion and conclusions
Significant limitations must be noted in interpreting the findings of this study. First, the two ways of classifying the subgroups are not the only options for categorization of substance of choice. In particular, the threshold used to classify study participants as users of particular drugs was low, and, although this threshold is arguably appropriate given that persons with psychotic illness may be vulnerable to adverse effects from only occasional substance use (4,32), clearer differences might have resulted if a higher threshold had been used.
Second, toxicologic confirmation of substances used was not obtained, and patients' reticence as well as lack of knowledge on the part of case managers may each contribute to inaccurate identification and categorization of substance of abuse. The extent of agreement between case managers and patients varied by substance, although the findings did not vary greatly according to whether they were based on case managers' or patients' self-reports of substance use. It has been suggested that multiple sources of classification have the greatest validity and reliability among persons with a dual diagnosis (3).
Third, the study sample was representative only of patients who were in contact with mental health services. Substantial numbers of persons with a dual diagnosis may have disengaged from care. Fourth, the significant findings indicate associations only, and no causal pathways can be inferred. Fifth, the number of patients in each subgroup was relatively small, which diminished statistical power. Finally, multiple statistical comparisons and the prominence of schizophrenia in this sample (73 percent) limit the interpretability of the results.
The study's primary analyses suggested a possible link between stimulant use and violence. This finding is similar to the findings of Mueser and colleagues (29) of an association between stimulant use and criminal behavior. Thus stimulant use may be a trigger to (45,46,47) or a marker of violence among persons with severe mental illness (48), which is relevant for risk assessment and also potentially for establishing service priorities. However, the apparent attenuation of this effect when the analysis was repeated with substance-use subgroups based on patients' self-reported use must be noted. This attenuation may have been due to sampling fluctuations in a reduced sample. Alternatively, it is possible that case managers were biased toward perceiving stimulant users as violent.
A lack of significant differences between subgroups or of marked trends toward such differences is the most striking aspect of our findings. The study limitations discussed above represent one set of potential reasons for this result. Alternatively, the aspects of substance use that have the greatest impact on the problems, needs, and service use of persons with severe mental illness may be common to a number of substances. For example, intoxication with alcohol or a variety of drugs might have similar effects on compliance, organization of activities, and self-care.
Another possible explanation is that the direct effects of substance misuse in this population may have contributed less to clients' generally severe clinical and social difficulties than did their psychotic illness and poor social circumstances. The relatively low levels of substance use but the high level of problems experienced—compared with those encountered in the specialist addictions services—lends plausibility to this explanation (4,32). Rather than being factors that directly worsen clinical and social problems, heavy drinking and drug use might be nonspecific markers for exacerbating factors, such as lack of other coping skills, social support and activity, and social exclusion and deprivation. If this explanation is correct, it strengthens the case for a broad approach to treating patients with a dual diagnosis, focusing not only on substance use but also on engaging clients, addressing social exclusion, and working to build coping strategies and supportive social networks. Finally, if substance of choice does not clearly define subgroups, it may be worth investigating whether severity of substance misuse might be a more useful means of distinguishing subgroups with different needs.
Acknowledgments
The authors thank Liz Brewin, B.Sc., R.M.N., Jane Marshall, M.R.C.Psych., Paul McCrone, Ph.D., Ian White, Ph.D., Kevin Gournay, Ph.D., C.B.E., Lindsay Denford, B.Sc., R.M.N., Shamil Wanigaratne, D.Clin.Psych., A.F.B.Ps.S., Jed Boardman, M.R.C.Psych., Tom Craig, M.B.B.S, Ph.D., Martin Knapp, Ph.D., and John Ba'lazs, M.R.C.G.P. Funding for this project was provided by the Bethlem and Maudsley National Health Service Trust.
Ms. Miles is a research assistant and Ms. Amponsah-Afuwape is a research worker with the COMO dual diagnosis project at the Institute of Psychiatry at Kings College London. Dr. Johnson is senior lecturer in the department of psychiatry and behavioral sciences of the Royal Free and University College London Medical Schools. Dr. Finch is consultant psychiatrist for the addictions directorate at the institute. Dr. Leese is senior lecturer in statistics and Professor Thornicroft is director of the health services research department at the institute. Professor Thornicroft is also head of community psychiatry (PRiSM) and consultant psychiatrist at the institute. Send correspondence to Ms. Miles at the Clinical Psychology Department, Institute of Psychiatry, Kings College London, De Crespigny Park, Denmark Hill, London SE5 8AF, United Kingdom (e-mail, [email protected]).
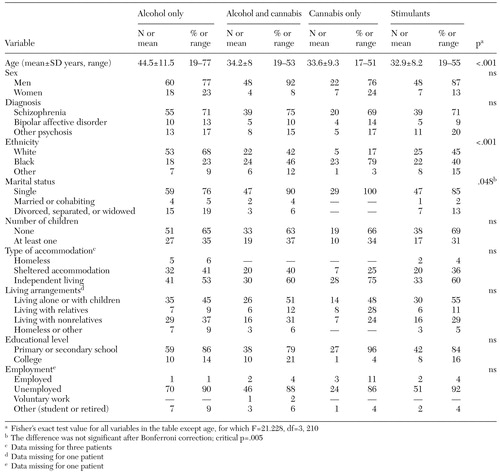 |
Table 1. Sociodemographic characteristics of 214 patients with a dual diagnosis, by substance-use subgroup
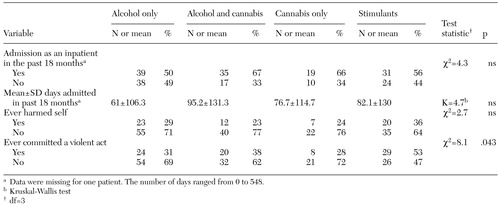 |
Table 2. Use of inpatient services and lifetime history of self-harm or violence in a sample of 214 patients with a dual diagnosis, by substance-use subgroup
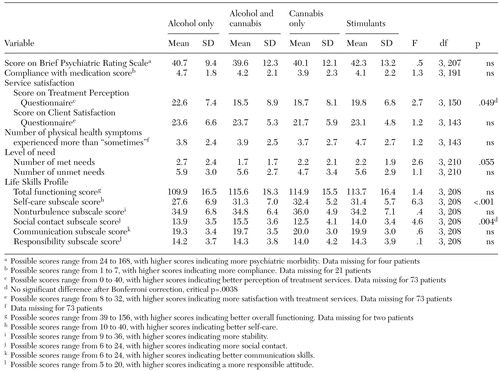 |
Table 3. Differences in physical and psychiatric symptoms, compliance with medication, service satisfaction, levels of need, and social functioning in a sample of 214 patients with a dual diagnosis, by substance-use subgroup
1. Drake RE, Brunette MF: Complications of severe mental illness related to alcohol and other drug use disorders, in Recent Developments in Alcoholism, vol 14: Consequences of Alcoholism. Edited by Galanter M. New York, Plenum, 1998Google Scholar
2. Barbee JG, Clark PD, Crapanzano MS, et al: Alcohol and substance abuse among schizophrenic patients presenting to an emergency psychiatric service. Journal of Nervous and Mental Disease 177:400-407, 1989Crossref, Medline, Google Scholar
3. Drake RE, Osher FC, Wallach MA: Alcohol use and abuse in schizophrenia: a prospective community study. Journal of Nervous and Mental Disease 177:408-414, 1989Crossref, Medline, Google Scholar
4. Mueser KT, Yarnold PR, Levinson DF, et al: Diagnostic and demographic correlates of substance abuse in schizophrenia and major affective disorder. Acta Psychiatrica Scandinavica 85:48-55, 1992Crossref, Medline, Google Scholar
5. Regier DA, Farmer ME, Rae DS, et al: Comorbidity of mental disorders with alcohol and other drug abuse. JAMA 264:2511-2518, 1990Crossref, Medline, Google Scholar
6. Cuffel BJ, Shumway M, Chouljian TL, et al: A longitudinal study of substance use and community violence in schizophrenia. Journal of Nervous and Mental Disease 182:704-708, 1994Crossref, Medline, Google Scholar
7. Bernadt MW, Murray RM: Psychiatric disorder, drinking, and alcoholism: what are the links? British Journal of Psychiatry 148:393-400, 1986Google Scholar
8. Duke P, Pantellis C, Barnes T: South Westminster Schizophrenia Survey: alcohol use and its relationship to symptoms, tardive dyskinesia, and illness onset. British Journal of Psychiatry 164:630-636, 1994Crossref, Medline, Google Scholar
9. Smith J, Frazer S, Boer H: Dangerous dual diagnosis patients. Hospital and Community Psychiatry 45:280-281, 1994Abstract, Google Scholar
10. Menezes P, Johnson S, Thornicroft G, et al: Drug and alcohol problems among individuals with severe mental illness in South London. British Journal of Psychiatry 169:334-337, 1996Crossref, Medline, Google Scholar
11. Cantwell R, Brewin J, Glazebrook C, et al: Prevalence of substance misuse in first episode psychosis. British Journal of Psychiatry 174:150-153, 1999Crossref, Medline, Google Scholar
12. Farrell M, Howes S, Taylor C, et al: Substance misuse and psychiatric comorbidity: an overview of the OPCS national psychiatric morbidity survey. Addictive Behaviours 23:909-918, 1998Crossref, Medline, Google Scholar
13. Wheatley M: The prevalence and relevance of substance use in detained schizophrenic patients. Journal of Forensic Psychiatry 9:114-129, 1998Crossref, Google Scholar
14. Wright S, Gournay K, Glorney E, et al: Dual diagnosis in the suburbs: prevalence, need, and inpatient service use. Social Psychiatry and Psychiatric Epidemiology 35:297-304, 2000Crossref, Medline, Google Scholar
15. Soyka M, Albus M, Kathmann M, et al: Prevalence of alcohol and drug abuse in schizophrenic inpatients. European Archives of Psychiatry and Clinical Neurosciences 242:362-372, 1993Crossref, Medline, Google Scholar
16. Fernandez Fernandez JA, Marquina Planchadell MM: El uso de drogas en personas con problemas psiquiatricos cronicos [Drug use among persons with chronic psychiatric problems]. Psicothema 7:557-567, 1995Google Scholar
17. Hambrecht M, Häfner H: Führen Alkohol und Drogenmissbrauch zur Schizophrenie? [Do alcohol or drug abuse induce schizophrenia?] Nervenarzt 67:36-45, 1996Google Scholar
18. Verdoux H, Mury M, Besanon G, et al: Etude comparative des conduites toxicomaniaques dans les trouble bipolaires, schizophréniques et schizoaffectifs [Substance abuse among persons with bipolar disorder, schizophrenia, and schizoaffective disorder]. L'Encéphale 22:95-101, 1996Medline, Google Scholar
19. Lambert M, Haasen C, Mass R, et al: Konsummuster und Konsummotivation des Suchtmittelgebrauchs bei schizophrenen Patienten [Consumption patterns and motivation for use of addictive drugs in schizophrenic patients]. Psychiatrische Praxis 27:185-189, 1997Google Scholar
20. Modestin J, Nussbaumer C, Angst D, et al: Use of potentially abusive psychotropic substances in psychiatric inpatients. European Archives of Psychiatry and Clinical Neurosciences 247:146-153, 1997Crossref, Medline, Google Scholar
21. Launay C, Petitjean F, Perdereau F, et al: Conduites toxicomaniaques chez les malades mentaux: une enquete en Ile-de-France [Addictive behaviors in mental disorders: a survey in the Paris area]. Annales Medico-Psychologiques 156:482-486, 1998Google Scholar
22. Weiss RD, Mirin SM, Frances RJ: The myth of the typical dual diagnosis patient. Community Psychiatry 43:107-108, 1992Google Scholar
23. Gournay K, Sandford T, Johnson S, et al: Dual diagnosis of severe mental health problems and substance abuse/dependence: a major priority for mental health nursing. Journal of Psychiatric and Mental Health Nursing 4:89-95, 1997Crossref, Medline, Google Scholar
24. Lehman A, Myers C, Dickson L, et al: Defining subgroups of dual diagnosis patients for service planning. Hospital and Community Psychiatry 45:556-561, 1994Abstract, Google Scholar
25. Zimberg S: A dual diagnosis typology to improve diagnosis and treatment of dual disorder patients. Journal of Psychoactive Drugs 31:47-51, 1999Crossref, Medline, Google Scholar
26. Shumway M, Cuffel B: Symptom heterogeneity in comorbid alcohol disorder. Journal of Mental Health Administration 23:338-347, 1996Crossref, Medline, Google Scholar
27. Lambert MT, Griffith JM, Hendrickse W: Characteristics of patients with a substance abuse diagnosis on a general psychiatry unit in a VA Medical Center. Psychiatric Services 47:1104-1107, 1996Link, Google Scholar
28. Mowbury CT, Ribis KM, Soloman M, et al: Characteristics of dual diagnosis patients admitted to an urban, public psychiatric hospital: an examination of individual, social, and community domains. American Journal of Drug and Alcohol Abuse 23:309-326, 1997Crossref, Medline, Google Scholar
29. Mueser KT, Essock SM, Drake RE, et al: Rural and urban differences in patients with dual diagnosis. Schizophrenia Research 48:93-107, 2001Crossref, Medline, Google Scholar
30. Westreich L, Guedj P, Galanter M, et al: Differences between men and women in dual diagnosis treatment. American Journal of Addictions 6:311-317, 1997Crossref, Medline, Google Scholar
31. Mueser KT, Yarnold PR, Levinson DF, et al: Prevalence of Substance Abuse in Schizophrenia: Demographic and Clinical Correlates. Schizophrenia Bulletin 16:31-56, 1990Crossref, Medline, Google Scholar
32. Drake RE, Essock SM, Shaner A: Implementing dual diagnosis services for clients with severe mental illness. Psychiatric Services 52:469-476, 2001Link, Google Scholar
33. Clayton R: Multiple drug use: epidemiology, correlates, and consequences, in Recent Developments in Alcoholism, vol 4. Edited by Galanter M. New York, Plenum, 1986Google Scholar
34. Kemp R, Hayward P, Applewhite G, et al: Compliance therapy in psychotic patients: a randomised control trial. British Medical Journal 312:345-349, 1996Crossref, Medline, Google Scholar
35. Slade M, Thornicroft G, Loftus L, et al: CAN: Camberwell Assessment of Need. London, United Kingdom, Gaskell, 1999Google Scholar
36. Rosen A, Hadzi-Pavlovic D, Parker G: The Life Skills Profile: a measure assessing function and disability in schizophrenia. Schizophrenia Bulletin 15:325-337, 1989Crossref, Medline, Google Scholar
37. Marsden J, Stewart D, Gossop M, et al: Assessing client satisfaction with treatment for substance use problems and the development of the Treatment Perceptions Questionnaire. Addiction Research 8:455-470, 2000Crossref, Google Scholar
38. Atkinsson CC, Zwick R: The Client Satisfaction Questionnaire: Psychometric Properties and Correlation With Service Utilization and Psychotherapy Outcome: Evaluation and Program Planning 6: Special Issue. Pergamon, New York, 1982Google Scholar
39. Saunders JB, Aasland OG, Babor TF, et al: Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons With Harmful Alcohol Consumption II. Addiction 88:791-804, 1993Crossref, Medline, Google Scholar
40. Marsden J, Gossop M, Stewart D, et al: The Maudsley Addiction Profile (MAP): a brief instrument for assessing treatment outcome. Addiction 93:1857-1867, 1998Crossref, Medline, Google Scholar
41. Lukoff D, Liberman RP, Nuechterlein KH: Manual for the expanded Brief Psychiatric Rating Scale: symptom monitoring in the rehabilitation of schizophrenic patients. Schizophrenia Bulletin 12:594-602, 1986Google Scholar
42. Beecham J, Knapp M: Costing psychiatric interventions, in Measuring Mental Health Needs. Edited by Thornicroft G. London, United Kingdom, Gaskell, 1992Google Scholar
43. Alcohol and the Heart in Perspective: Sensible Limits Reaffirmed. London, United Kingdom, Royal College of Physicians and Royal College of Psychiatrists and General Practitioners, 1995Google Scholar
44. Salyers MP, Mueser KT: Social functioning, psychopathology, and medication side effects in relation to substance use and abuse in schizophrenia. Schizophrenia Research 48:109-123, 2001Crossref, Medline, Google Scholar
45. Honer WG, Gewirtz G, Turey M: Psychosis and violence in cocaine smokers. Lancet 2:451, 1987Crossref, Medline, Google Scholar
46. Cordess C, Murray K: Forensic aspects of "crack" misuse. Medicine, Science, and the Law 31:249-250, 1991Crossref, Medline, Google Scholar
47. Forshaw DM, Strang J: Drugs, aggression, and violence, in Violence in Society. Edited by Taylor P. London, United Kingdom, Royal College of Physicians, 1993Google Scholar
48. Scott H, Johnson S, Menezes P, et al: Substance misuse and risk of aggression and offending amongst the severely mentally ill. British Journal of Psychiatry 172:345-350, 1998Crossref, Medline, Google Scholar



