Patterns of Antidepressant Use Among Children and Adolescents
Abstract
OBJECTIVE: The purpose of this study was to identify patterns of new antidepressant use among children and adolescents and to determine whether the duration of treatment was sufficient. METHODS: A retrospective 12-month analysis was conducted of claims data for a cohort of nine- to 18-year-old new users of antidepressants in an Ohio Medicaid population. Treatment duration was categorized into five time intervals reflecting initial treatment through various continuation periods. RESULTS: A total of 554 children and adolescents started an antidepressant regimen during a three-month period. These children were mostly Caucasians (78 percent), and their average age was 13 years. Boys and girls were equally represented. The use of antidepressants increased with age among girls but declined among boys. The distribution of antidepressants dispensed was selective serotonin reuptake inhibitors, 47 percent; tricyclic antidepressants, 27 percent; and other antidepressants, 23 percent. The specific agent used varied by primary psychiatric diagnosis. The proportion of children who completed treatment was 94 percent for the four-week treatment period, 23.5 percent for the six-month period, and 12.6 percent for the whole year. CONCLUSIONS: Antidepressants are used in the treatment of children and adolescents who have a wide array of mental health problems. As with adults, continuation of treatment among children and adolescents declines dramatically after an initial period. In addition to studies of the clinical efficacy of antidepressant use among children and adolescents, future research is needed to assess adherence to practice guidelines and health outcomes in childhood and adolescent mental health.
An estimated 20 percent of children have a mental illness that causes at least mild functional impairment, but only 5 percent receive any kind of mental health care (1,2,3). Antidepressant medications are among the psychotropic medications most commonly prescribed for children and adolescents, and their use is increasing dramatically (4). In 1993-1994, children below the age of 18 years were six times as likely as in 1985 to receive a prescription for an antidepressant; between 1990 and 1996, the total number of prescriptions of selective serotonin reuptake inhibitors (SSRIs) for children and adolescents increased by 69 percent (5,6,7). Despite this dramatic increase in use, there is little understanding of what mental health conditions these agents are being used to treat. It is possible that their use is outpacing existing clinical evidence of their efficacy.
Evidence from naturalistic studies in adult populations indicates that antidepressants are commonly used inappropriately (8,9,10,11). These studies have documented incorrect dosages and insufficient duration of therapy. Continuation of antidepressant therapy is recommended to minimize high rates of relapse and recurrence of depressive symptoms (12,13). Practice guidelines for treating childhood and adolescent mental disorders other than depression indicate that these conditions may also require long-term treatment with antidepressants (4,14,15,16,17,18,19,20,21).
Patterns of antidepressant use among children and adolescents have not been documented. The purpose of this study was to fill a gap in the research on patterns of new antidepressant use among children and adolescents. First, we assessed the current state of practice by studying a cohort of new antidepressant users, including analyses of differences in use by race, age by gender, and primary mental health diagnosis. Second, we assessed whether treatment duration was insufficient in this cohort, as has been documented for adults.
Methods
This study was a retrospective cohort analysis of 1997-1998 claims data obtained from the Ohio Department of Human Services. Patients were selected from the claims records of continuously enrolled fee-for-service Medicaid recipients aged nine to 18 years. The cohort consisted of patients for whom antidepressant treatment was initiated during a three-month period—August 1 to October 31, 1997—and who had had no claims for an antidepressant prescription in the preceding seven months—January 1 through July 31, 1997. The protocol was reviewed and approved by the institutional review board of the University of Cincinnati Medical Center.
Children who lived in an institutional setting, those who had had a medical or institutional visit for depression in the three months before antidepressant therapy was initiated, and those who had received a diagnosis of enuresis or obsessive-compulsive disorder during the seven months before initiation of treatment were excluded. Depression, enuresis, and obsessive-compulsive disorder were identified by screening institutional and medical claims for ICD-9 codes (22).
Baseline demographic data on the study children included age, living arrangement (family home, adoptive assistance, or foster care), race (Caucasian, African American, or other), and gender. To identify psychiatric conditions, we screened institutional and medical claims from the first ten months of 1997 for concomitant mental disorders as indicated by ICD-9 codes. (A table of the codes used to identify these diagnoses is available from the first author.) This period included the time up to and including the initiation of antidepressant therapy.
We identified the first antidepressant prescribed for each of the children during the index period. Antidepressants were classified as tricyclic antidepressants, SSRIs, monoamine oxidase inhibitors (MAOIs), or other antidepressants (bupropion, trazodone, venlafaxine, nefazodone, or mirtazapine). Using the date of the index antidepressant prescription as the treatment start date for each individual, we determined each child's antidepressant prescriptions for the next 12 months.
Duration of antidepressant therapy
We selected five time intervals for the purpose of assessing continuity of treatment. We set the first treatment period at four weeks to allow us to determine whether the children had received sufficient medication for an initial trial period. The second period was set at six weeks to allow us to determine how many children proceeded past the trial period. The next three periods were designed to enable us to examine longer-term treatment extending to six months, nine months, and a year.
For each treatment period, we developed an algorithm to identify prescriptions received and to estimate the number of days of therapy associated with those prescriptions. (A copy of the complete algorithm is available on request.) The second step of the algorithm required that we establish whether the children received enough medication to reach the end of each designated treatment period. Because Ohio Medicaid prescription claims data do not indicate the duration of treatment (days' supply) for each prescription, we imputed days' supply for each prescription by using three methods. Each method incorporated the Ohio Medicaid rule that limits medication prescriptions to a 34-day supply.
We first determined days' supply on the basis of drug quantity. For any quantity up to a 34-day supply, days' supply was set at that quantity. For quantities in excess of a 34-day supply, days' supply was set at 30 days on the basis of Medicaid's restriction. For almost 79 percent of the prescriptions, the quantities were multiples of 30, typically reflecting a 30-day supply of medication at multiple doses per day—for example, a quantity of 60 would indicate a twice-daily dosage for 30 days. Prescriptions for quantities of less than a 30-day supply accounted for another 20 percent of the prescriptions. Prescriptions for liquid dosage forms were assumed to last for 30 days.
To test the sensitivity of prescription quantity as an indicator of days' supply, we imputed days' supply for each prescription by using two types of dosage adjustments. The first and simpler approach involved the average recommended daily dose for each drug, as listed in Table 1 (16,23,24,25,26,27). With the second type of adjustment, because the ages of the children varied and because recommended dosages vary by weight among children, we estimated age- and gender-adjusted dosages for each drug in order to estimate minimum and maximum daily doses (dosage table available on request). These dosage ranges are also shown in Table 1. Age- and gender-based weights were taken from the Centers for Disease Control's stature-for-age and weight-for-age charts (28). With both methods, we summed days' supply for each treatment period to determine whether the child had received enough medication to last until the end of the treatment period.
All measures of days' supply were adjusted in the event of duplicate therapy. If a child or adolescent was simultaneously receiving more than one antidepressant as a result of overlapping dates of service and days' supply, we eliminated the days' supply variables for one of the medications. This approach allowed the prescription to contribute toward the number of prescriptions without affecting days' supply.
Analysis
All the data are descriptive. We compared the study population with the sampling frame—Medicaid enrollees in the same age group who were not receiving antidepressants—to determine how antidepressant use varied by race, age, and gender. We then examined the distribution of types of antidepressants to determine initial selection as well as use over the course of the year. We also analyzed the relationship between the initial antidepressant and the presence of predominant mental illnesses. Finally, we estimated the duration of antidepressant treatment.
Results
Across the continuous enrollment period, we identified 68,386 children and adolescents in the Ohio Medicaid population. A total of 6,911 of these children (10.6 percent) received at least one antidepressant. During the three-month index period, 752 children started an antidepressant regimen, but only 554 met all the study's inclusion criteria. Of the children excluded, 135 had used antidepressants in the previous seven months, 38 had enuresis, 23 were institutionalized, and 12 had obsessive-compulsive disorder. A few children were excluded for more than one reason.
A total of 412 of the antidepressant users (74 percent) lived in their family home, and the remaining 142 lived either in foster care or under adoptive assistance arrangements. Other demographic characteristics of the new antidepressant users are summarized in Table 2. A majority of the children were Caucasians, whose use of antidepressants was disproportionately high compared with that of the Medicaid reference population. The study cohort contained nearly equal proportions of boys and girls, consistent with the proportions in the reference population. Both the boys and the girls in the study cohort were significantly older than those in the reference population, and antidepressant use increased significantly with age among girls but not among boys. The percentage of new male users was highest for boys younger than 14 years and then declined to single digits among 16-year-olds. The use of antidepressants among girls increased with age and peaked at age 15.
As can be seen from Table 3, the children in this study received mostly SSRIs as their initial therapy; tricyclic antidepressants were the second most frequently prescribed agents. A few patients started with a combination of antidepressants, most of which included trazodone combined with either an SSRI or a tricyclic antidepressant. Sertraline was the most common SSRI for initial therapy, followed by fluoxetine and paroxetine. Imipramine was the most common tricyclic antidepressant. Bupropion was the main other type of agent selected as initial therapy. None of the children received prescriptions for MAOIs. Dispensing patterns across the year were similar to those for initial therapy, except that there was a slight decline in the use of tricyclic antidepressants and a slight increase in the use of other agents.
The most prevalent mental disorder noted during the periods before and during initiation of treatment was attention-deficit hyperactivity disorder (ADHD), followed by adjustment reactions, emotional disorders, and conduct disorder, as shown in Table 4. Forty-two children (7.6 percent) had a medical or institutional claim that indicated a suicide attempt. Among the children with ADHD, tricyclic antidepressants were most commonly used (39.1 percent), followed closely by SSRIs (38.1 percent). SSRIs were more common in the other major diagnostic categories, but their rates of use varied.
Data on the duration of therapy are summarized in Table 5. In the first four weeks of treatment, the average number of prescriptions received was 1.6. After two more weeks, the mean number of prescriptions was almost two, which suggests that most of the children received a second prescription during the initial period of treatment. Within a six-month treatment period, the children received fewer than four prescriptions on average. The gap was wider for the nine-month period, during which the children received an average of about five prescriptions; over the course of the full year, the average number of prescriptions received was about six.
Table 5 includes days' supply calculated on the basis of the number of prescriptions received during each treatment interval. Days' supply calculated on the basis of quantity and maximum days' supply adjusted for age and gender were nearly identical. For the four-week treatment period, the children received on average enough medication to cover 45 days. Over the course of the year, they received less than a six-month supply of medication (164 days). Use of these two methods gave us the upper limit, or best-case scenario, of days' supply calculated by either incorporating the parameter of minimum daily dose recommendations or relying on the maximum number of days allowed under Medicaid rules.
The age- and gender-adjusted minimum days' supply (based on the maximum daily dose) was used to calculate the minimum duration of therapy. Less than a two-week supply of medication was received for the four-week treatment period and the six-week treatment period; a one-month supply was received for the six-month treatment period; and less than a two-month supply was received for the nine- and 12-month treatment periods. Average days' supply as calculated from the average daily dose (data not shown) provided an intermediate number of days. For example, for the six-week treatment period, a 31-day supply of antidepressant medication was received, based on an average daily dose. For the entire year and based on the average daily dose, days' supply was 110 days.
Over the course of the year the proportion of children whose therapy lasted until the end of each of the five treatment intervals declined dramatically. Given the similarity in days' supply calculated on the basis of quantity and days' supply adjusted for age and gender, we report the quantity-based data here. The proportions of children who reached the end of each treatment period were 94 percent for the four-week period, 58.5 percent for the six-week period, 23.5 percent for the six-month period, 14.8 percent for the nine-month period, and 12.6 percent for the entire year.
Discussion
The purpose of this study was to fill a gap in the existing literature on patterns of new antidepressant use among children and adolescents. Most of the children and adolescents in our cohort received an SSRI; tricyclic antidepressants were the second most commonly prescribed agents. The use of antidepressants was more prevalent among Caucasians and among girls in their upper teen years. The type of antidepressant differed according to the underlying psychiatric diagnosis.
Although we did not directly link the initiation of antidepressant therapy with diagnosis, the limited clinical evidence showed that the antidepressants used by children with different comorbid conditions were fairly consistent. More than 62 percent of the children who had a diagnosis of depression received an SSRI, which is consistent with the recommendations of the American Academy of Child and Adolescent Psychiatry (AACAP) (16). SSRIs may also be effective for treating anxiety symptoms, such as social phobia, separation anxiety, and panic disorder (17). Our data showed that of the children with anxiety, 70 percent received an SSRI. Tricyclic antidepressants and bupropion may decrease hyperactivity and aggressive symptoms associated with ADHD (20,29,30,31), but our results were mixed for the children with ADHD in this cohort, for whom the use of tricyclic antidepressants and SSRIs was almost equal. Because no medications have consistently demonstrated effectiveness in the treatment of conduct disorder, antidepressants are recommended only for use in controlling specific symptoms (1,19). The children in this study who had conduct disorder received primarily SSRIs (48.4 percent). Likewise, about 50 percent of the children with emotional disorders and about 60 percent of those with adjustment reaction diagnoses received an SSRI.
We initially intended to identify new cases of depression in an ambulatory population of children and adolescents. We therefore excluded children who lived in institutional settings in the three months before initiation of treatment and those who had received a diagnosis of depression, enuresis, or obsessive-compulsive disorder in the seven months before initiation of treatment. Twenty-three institutionalized children were excluded, because their continued use of therapy would have been more likely to be directly controlled than in a noninstitutionalized population.
At the time of sample selection, antidepressants were indicated for use only among children with obsessive-compulsive disorder, enuresis, or depression. We expected that by excluding obsessive-compulsive disorder and enuresis, we would identify a population of children with depression who were being newly treated with antidepressants. Only 50 cases of obsessive-compulsive disorder and enuresis were noted in our sample, so although use of antidepressants for these disorders is legitimate, the exclusion of children with these disorders should not have seriously affected our results.
In fact, the study design meant that children with depression were underrepresented, and the results highlight the broad array of diagnoses that have been treated with antidepressants. Thus these selection criteria shifted the focus of the study to describing a broader range of mental illnesses that may be managed with antidepressant therapy and a better description of a standard of care not previously reported. In addition, it seems that there are a number of children who are receiving antidepressants without any clearly documented indication of the need for such treatment: nearly a quarter of the new antidepressant users in this cohort did not have any diagnosis of mental illness recorded during the baseline period.
Very few of the children in this cohort had an adequate duration of therapy as recommended in AACAP's guidelines for pediatric depression (14,16). These findings are consistent with the low rates of therapy continuation documented for adults (11 to 30 percent) on the basis of similar methods (8,9,10,11). Teenagers for whom antidepressants are prescribed may actually require a slightly longer acute trial of medication—eight to 12 weeks—before a conclusion can be made about whether therapy is suboptimal in producing relief of symptoms (14). Although we used depression guidelines to establish the five treatment intervals used in this study, AACAP guidelines for conduct disorder, ADHD, anxiety, and bipolar disorder also indicate that these conditions may require long-term treatment (17,18,19,20,21). Given the preponderance of ADHD in our cohort, we repeated the analysis of treatment continuation but excluded the children with ADHD, and the results were nearly identical.
There are alternative approaches for estimating treatment compliance on the basis of claims data. Previous studies have defined adherence to antidepressant therapy as the filling of four prescriptions in a six-month period or as the filling of six prescriptions in a 12-month period (8,9,10). On the basis of these definitions, the children in this study would generally have been classified as adherent, because they filled an average of 3.8 prescriptions in a six-month period and 5.8 prescriptions during the course of a year. By analyzing days' supply of medication for set treatment periods, we showed that only 23.5 percent of our study population received enough medication for a six-month treatment period and that only 12.6 percent received enough for a year's treatment. Simply counting the number of prescriptions filled would likely overstate the level of compliance.
The results of this analysis have limited generalizability given the source of the data. Medicaid, by definition, includes persons from lower socioeconomic groups. Although this fact limits the ability to apply our findings to other populations, our study may represent a worst-case scenario because of problems in follow-up care among Medicaid recipients.
Conclusions
Despite inconclusive clinical evidence of efficacy, antidepressants are used in the treatment of children and adolescents who have a wide array of mental health problems. We have provided an overview of the types of agents used and the demographic characteristics of the population using them. Caucasians were more likely to receive an antidepressant than other racial groups. Age and gender trends showed greater use among younger males and among females in their upper teens. SSRIs dominated treatment choice for most mental health diagnoses except ADHD.
In addition, continuation of treatment declined dramatically after an initial four- to six-week treatment period. It is possible that the clinicians of the children in this study thought that the medications were not effective and pursued other forms of treatment. Alternatively, the child or the child's family may have chosen not to continue treatment for a variety of reasons, including adverse events and inconvenience. In addition to studies evaluating the clinical efficacy of antidepressants for children and adolescents, future research is needed to assess racial and gender-by-age differences and how adherence to practice guidelines relates to child and adolescent mental health outcomes.
Acknowledgments
This project was supported by a grant from the Ohio Department of Human Services and the Ohio Board of Regents through the Medicaid Technical Assistance and Policy Program.
Dr. Shireman is assistant professor of pharmacoeconomics in the pharmacy practice department of the University of Kansas School of Pharmacy, 1251 Wescoe Hall Drive, Lawrence, Kansas 66045-7582 (e-mail, [email protected]). Dr. Olson is a research scientist at the Center for Health Outcomes and Pharmaco Economic Research at the University of Arizona College of Pharmacy in Tucson. Dr. Dewan is director of the Center for Quality Innovation and Research and assistant professor of psychiatry at the University of Cincinnati in Ohio. Preliminary results of this study were presented at the annual meeting of the American Pharmaceutical Association held March 5-9, 1999, in San Antonio.
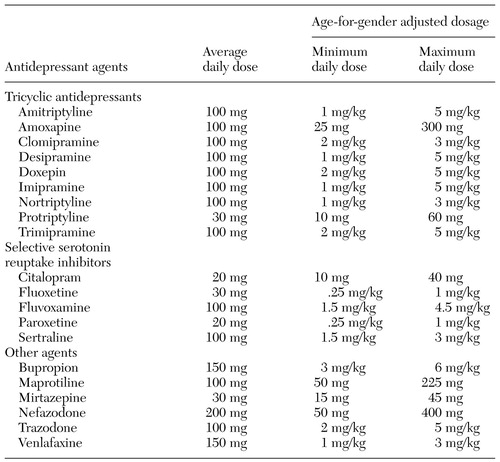 |
Table 1. Recommended antidepressant dosages for children and adolescents
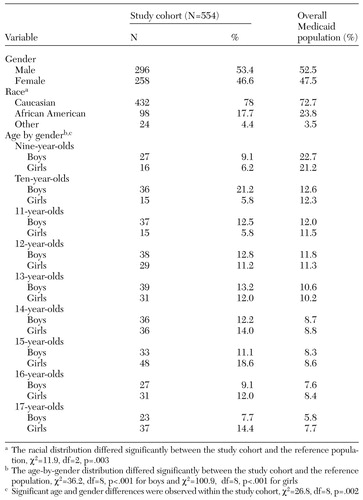 |
Table 2. Demographic characteristics of a cohort of new users of antidepressants in a Medicaid population in Ohio compared with the reference Medicaid population
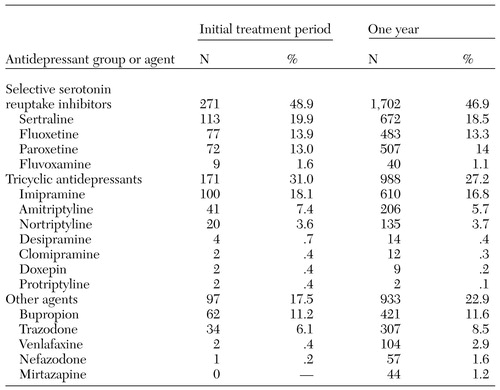 |
Table 3. Distribution of antidepressants initially dispensed and those dispensed throughout the study year to 554 children and adolescents
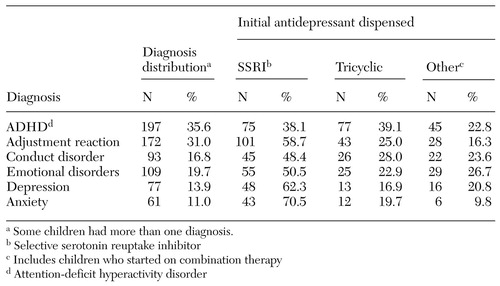 |
Table 4. Distribution of psychiatric diagnosis and initial antidepressant dispensed to 554 children and adolescents
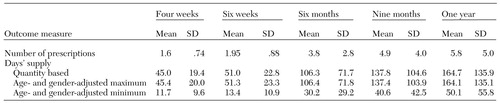 |
Table 5. Results of baseline and sensitivity analyses of duration of antidepressant treatment among children and adolescents
1. US Department of Health and Human Services: Mental Health: A Report of the Surgeon General. Bethesda, Md, National Institutes of Health, National Institute of Mental Health, 1999. Available at http://www.surgeongeneral.gov/library/mentalhealth/chapter3/sec1.htmlGoogle Scholar
2. Costello EJ, Burns BJ, Angold A, et al: How can epidemiology improve mental health services for children and adolescents? Journal of the American Academy of Child and Adolescent Psychiatry 32:1106-1114, 1993Google Scholar
3. McGee R, Feehan M, Williams S, et al: DSM-III disorders in a large sample of adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 29:611-619, 1990Crossref, Medline, Google Scholar
4. Emslie GJ, Walkup JT, Pliszka SR, et al: Nontricyclic antidepressants: current trends in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 38:517-528, 1999Crossref, Medline, Google Scholar
5. Strauch B: Use of Antidepression Medicine for Young Patients Has Soared. New York Times, Aug 10, 1997, p 1Google Scholar
6. Olfson M, Marcus SC, Pincus HA, et al: Antidepressant prescribing practices of outpatient psychiatrists. Archives of General Psychiatry 55:310-316, 1998Crossref, Medline, Google Scholar
7. Renaud J, Axelson D, Birmaher B: A risk-benefit assessment of pharmacotherapies for clinical depression in children and adolescents. Drug Safety 20:59-75, 1999Crossref, Medline, Google Scholar
8. Prien RF, Kocsis JH: Long-Term Treatment of Mood Disorders in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven, 1995Google Scholar
9. Thase ME: Maintenance treatments of recurrent affective disorders. Current Opinions in Psychiatry 6:16-21, 1993Crossref, Google Scholar
10. Kutcher S: Practitioner review: the pharmacotherapy of adolescent depression. Journal of Child Psychology and Psychiatry and Allied Disciplines 38:755-767, 1997Crossref, Medline, Google Scholar
11. Depression Guideline Panel: Depression in Primary Care: Detection, Diagnosis, and Treatment: Quick Reference Guide for Clinicians, no 5. AHCPR pub 93-0552. Rockville, Md, US Agency for Health Care Policy and Research, 1993Google Scholar
12. American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children and adolescents with depressive disorders. Journal of the American Academy of Child and Adolescent Psychiatry 37(10 suppl):63S-83S, 1998Google Scholar
13. American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children and adolescents with anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry 36(10 suppl):69S-84S, 1997Google Scholar
14. American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children and adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry 36:138-157, 1997Crossref, Medline, Google Scholar
15. American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children and adolescents with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry 36(10 suppl):122S-139S, 1997Google Scholar
16. American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 36(10 suppl):85S-121S, 1997Google Scholar
17. Keller MB, Lavori PW, Wunder J, et al: Chronic course of anxiety disorders in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 31:595-599, 1992Crossref, Medline, Google Scholar
18. Croghan TW, Melfi CA, Dobrez DG, et al: Effect of mental health specialty care on antidepressant length of therapy. Medical Care 37(4 suppl):AS20-AS23, 1999Google Scholar
19. Hylan TR, Crown WH, Meneades L, et al: SSRI antidepressant drug use patterns in the naturalistic setting: a multivariate analysis. Medical Care 37(4 suppl):AS36-AS44, 1999Google Scholar
20. Katon W, von Korff M, Lin E, et al: Adequacy and duration of antidepressant treatment in primary care. Medical Care 30(1):67-76, 1992Google Scholar
21. Melfi CA, Chawla AJ, Croghan TW, et al: The effects of adherence to antidepressant treatment guidelines on relapse and recurrence of depression. Archives of General Psychiatry 55:1128-1132, 1998Crossref, Medline, Google Scholar
22. St Anthony's ICD-9-CM Code Book, vol 1-3. Edited by Hart AC, Parvis SR. Reston, Va, St Anthony Publishing, 1998Google Scholar
23. Green WH: Child and Adolescent Clinical Psychopharmacology. Baltimore, Williams & Wilkins, 1995Google Scholar
24. Kutcher S: Child and Adolescent Psychopharmacology. Philadelphia, Saunders, 1997Google Scholar
25. Taketoma C, Hodding J, Kraus D: Pediatric Dosage Handbook. Cleveland, Oh, American Pharmaceutical Association, Lexi-Comp, 1998Google Scholar
26. Walsh BT: Child Psychopharmacology: Review of Psychiatry. Washington, DC, American Psychiatric Press, 1998Google Scholar
27. Werry JS, Aman MG: Practitioner's Guide to Psychoactive Drugs for Children and Adolescents. New York, Plenum, 1999Google Scholar
28. Centers for Disease Control and Prevention, National Center for Health Statistics: Clinical Growth Charts. Available at http://www.cdc.gov/growthchartsGoogle Scholar
29. Barrickman LL, Perry PJ, Allen AJ, et al: Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 34:649-657, 1995Crossref, Medline, Google Scholar
30. Conners CK, Casat CD, Gualtieri CT, et al: Bupropion hydrochloride in attention deficit disorder with hyperactivity. Journal of the American Academy of Child and Adolescent Psychiatry 35:1314-1321, 1996Crossref, Medline, Google Scholar
31. Zito JM, Safer DJ, dosReis S, et al: Trends in the prescribing of psychotropic medications to preschoolers. JAMA 283:1025-1030, 2000Crossref, Medline, Google Scholar



