Variation in Psychotropic Medication Prescription for Adults With Schizophrenia in the United States
Abstract
Objective:
Variation in prescription of psychotropic medications to patients with schizophrenia spectrum disorders may underlie health inequities. Using a national U.S. Medicaid sample, the authors examined prescription patterns of psychotropic medications commonly used for managing schizophrenia.
Methods:
Data from the 2011–2012 Medicaid Analytic eXtract were examined for demographic predictors of and variation across states in psychotropic medication prescription among adult patients diagnosed as having schizophrenia spectrum disorders (N=357,914). Percentages of patients in each state who filled prescriptions of at least 15 days of any antipsychotic, clozapine, antidepressant, benzodiazepine, mood stabilizer, or long-acting injectable (LAI) antipsychotic medication were determined after adjustment for demographic and clinical covariates. Multivariate regressions of clinical and demographic factors predicting prescription patterns were conducted.
Results:
Prescribing patterns for all types of psychotropic medications varied across states. Clozapine and LAI prescriptions showed the most dramatic differences across states and among patients with different demographic characteristics. Across states, adjusted proportions of prescriptions ranged from 4% to 22% for LAIs and from 1% to 11% for clozapine. Non-Hispanic Blacks and people of other race-ethnicities were more likely than non-Hispanic Whites to fill prescriptions for LAIs, and non-Hispanic Whites were more likely than individuals from other racial-ethnic groups to fill prescriptions for clozapine and all other medications.
Conclusions:
Considerable variation in prescribing patterns of LAIs and clozapine by race-ethnicity and across states suggests uneven quality of care for individuals with schizophrenia spectrum disorders in the United States. A better understanding of what causes this variation could inform policy makers to improve treatment for this vulnerable population.
HIGHLIGHTS
This nationwide study reports variation in prescribing patterns for major psychotropic medication classes used to treat patients with schizophrenia.
Significant variation was found among states in prescription of each psychotropic medication class, with 10-fold and fivefold variations in clozapine and long-acting injectable (LAI) antipsychotic medications, respectively.
Compared with non-Hispanic Whites, individuals from other racial-ethnic groups were more likely to fill prescriptions of any oral and LAI antipsychotic medications, and non-Hispanic Whites were more likely to fill prescriptions for clozapine and all other medications.
A better understanding of the reasons for the divergence of clozapine and LAI prescriptions by race-ethnicity is needed to identify the causes of these inequities.
Several classes of psychotropic medications are commonly prescribed to people with schizophrenia spectrum disorders, but only antipsychotic medications have strong evidence for effectiveness in treating such patients (1, 2). Among these medications, clozapine and long-acting injectable (LAI) formulations have distinctive roles. Clozapine is the established medication of choice for treatment-resistant schizophrenia and is the only drug approved for this indication (3). LAI antipsychotic medications are important options to help ensure medication adherence (4), but their benefits and how they are used are controversial (5, 6). Evidence for an important role for antidepressants in schizophrenia treatment has been increasing (1, 7), whereas mood stabilizers and benzodiazepines have limited evidence for effectiveness in this disorder (8–12).
Treatment variation refers to differences in treatment that are not attributable to differences in clinical presentation for persons with the same condition. Variation in treatment patterns is thought to reflect professional uncertainty—a lack of consensus or evidence regarding the best treatment choice for a condition (13). Other factors contributing to such variation are uncertainty of sequential timing of treatments, differences in availability of providers and services, and provider preferences (13, 14). Regulations, variations in health insurance benefits, pharmacy benefit management, and consumer preferences are other possible contributors (13, 14). In some cases, treatment variations may reflect inequities due to socioeconomic status, race, or ethnicity.
In a review of the literature, variations in prescription of psychotropic medications for schizophrenia have been documented despite treatment guidelines designed to reduce this variation (15). These studies provide evidence of variation by geographic location (16), within specific U.S. states (17–19), for specific medications (clozapine vs. other antipsychotic medications) (20) and medication classes (antidepressants vs. anxiolytics), or by demographic characteristics (African Americans vs. non-Hispanic Whites) (21). Previous studies, however, have not provided a portrait of variation across the major psychotropic medication classes used to treat patients with schizophrenia in the United States.
This study of variations in psychotropic medication prescriptions for adults diagnosed as having schizophrenia spectrum disorders extends these previous investigations. In addition to antipsychotic medications, we included antidepressants, mood stabilizers, and benzodiazepines because of clinical interest regarding the appropriate role of these medication classes in the management of schizophrenia (2, 7, 22, 23). We analyzed Medicaid claims data from 47 states and the District of Columbia with two complementary goals: to identify patient-specific factors that predict prescription of these medications and to determine whether variation in psychotropic medication prescription rates across U.S. states remains significant after controlling for clinical, demographic, and service use factors.
Methods
Data Source
The data for this study came from the 2011–2012 national Medicaid Analytic eXtract Inpatient, Other Services, Prescription Drug and Personal Summary data sets; Hawaii, Idaho, and Maine were excluded because of missing data. Data from individuals dually covered by Medicaid and Medicare were excluded, but we included data from individuals covered by Medicaid fee-for-service (FFS) and managed care plans. The institutional review board of New York State Psychiatric Institute approved this study.
Cohort Selection
We limited the final analytic sample to data from patients with one inpatient or two outpatient diagnoses of schizophrenia spectrum disorders (ICD-9-CM 295.xx), hereafter referred to as schizophrenia, by using the principal and second listed diagnosis codes in the 2011 Inpatient and Other Services data sets, a previously validated method (24). To improve interpretation of prescription of mood stabilizers, which are often used as anticonvulsant medications, data from patients with 2011 principal and second listed diagnoses of epileptic disorders (ICD-9-CM 345.xx) were excluded. The sample was limited to adults ages 18–64 years who filled at least a 15-day prescription of at least one oral psychotropic medication or prescription of an LAI medication and who were eligible for Medicaid for all months of 2012 (N=357,914).
Analysis of Prescription Patterns
The outcomes of interest were prescribing patterns for six types of psychotropic medications (see table in the online supplement to this article) by state in 2012: any antipsychotic, clozapine, LAI antipsychotic medication, antidepressant, mood stabilizer, and benzodiazepine medication. Filled prescriptions were defined as at least a 15-day supply of any oral antipsychotic, antidepressant, benzodiazepine, or mood stabilizer or any LAI antipsychotic medication. To examine practice variation in psychotropic medication prescription by state, indicator variables of each state were the primary predictor of interest in these analyses.
Demographic, Clinical, and Service Use Covariates
Because our sample included Medicaid FFS and managed care plans that could affect prescribing patterns, we included a covariate indicating the financing arrangement (FFS, managed care, or other). Demographic covariates included age, sex, race-ethnicity (non-Hispanic Black; non-Hispanic White; Hispanic; and an “other” race category including American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, more than one race, or unknown). Clinical covariates included previous year (2011) schizoaffective subtype (defined as >50% of diagnosis code 295 coded as 295.7x) or schizophreniform subtype (defined as >50% of diagnosis code 295 coded as 295.4x) and psychiatric comorbid conditions (intellectual disability, substance use disorders, depression, anxiety disorders, or self-harm) (see table in the online supplement). Clinical covariates also included previous year (2011) comorbid general medical conditions that may affect antipsychotic or other treatment choices, such as diabetes; cardiovascular disease, excluding essential hypertension diagnosis code 401.xx (25–27); white blood cell disease; intracranial injury (28); hyperlipidemia; drug-induced dyskinesia (29); and HIV (30) (see table in the online supplement). Previous year (2011) service uses, including psychiatric outpatient visits, any mental health emergency department visits, and inpatient psychiatric admissions (see table in the online supplement), were also included as covariates.
Analysis Strategy
Medicaid programs differ among states in the demographic characteristics of the Medicaid-covered populations and in covered mental health and other medical services. We examined variation in psychotropic medication prescription by state, controlling for individual-level demographic, clinical, and service use characteristics and financing arrangement. Logistic regressions with filled prescriptions of each psychotropic class (yes or no) as the outcome were predicted by using dummy variables for each state and all other covariates. We included a Bonferroni adjustment for multiple comparisons of these six models, with p<0.008 (0.05 divided by 6) or a confidence interval (CI) of 99.2% (hereafter rounded to 99%). SAS, version 9.4 (31), was used to conduct these analyses. Odds ratios (ORs) and 99% CIs assessed the association of each demographic and clinical covariate. We calculated adjusted proportions of prescriptions for each medication for each state from the logistic regressions (by using the SAS Proc GENMOD with a binomial distribution and logit link) as the overall population mean values adjusted for all other covariates. Tests of variation in filled prescriptions among states were performed with the chi-square likelihood ratio test from the fully adjusted logistic regression models.
Results
Sample Characteristics
Table 1 shows the general characteristics of the study population, providing context for our analyses of state variation in prescribing patterns. The composition of the sample was 49% women, 35% non-Hispanic Black, 40% non-Hispanic White, and 12% Hispanic. Patients with schizoaffective disorder made up 33% of the sample, and <1% of patients had schizophreniform disorder. Past-year depression (34%) was the most common comorbid mental health condition, and cardiovascular disease (35%), hyperlipidemia (24%), and diabetes mellitus (21%) were the most common comorbid general medical conditions. Almost 61% of the patients had nine or fewer outpatient visits during 2011, 26% had a mental health emergency department visit, and 81% had no past-year psychiatric inpatient admissions. Almost 70% of patients had FFS, and 31% had managed care financing arrangements. Only 30% of patients were prescribed psychotropic monotherapy, and the highest rates of polypharmacy prescription were for two types of medications (38%), highlighting the need to study other psychotropic medications in addition to antipsychotic medications commonly prescribed for schizophrenia.
| Characteristic | N | % |
|---|---|---|
| Age (M±SD years) | 43.2±12.6 | |
| Women | 174,272 | 48.7 |
| Race-ethnicity | ||
| White, non-Hispanic | 141,261 | 39.5 |
| Black, non-Hispanic | 125,256 | 35.0 |
| Hispanic or Latino | 41,262 | 11.5 |
| Otherb | 50,135 | 14.0 |
| Schizophrenia subtypes | ||
| Schizoaffective | 118,930 | 33.2 |
| Schizophreniform | 2,007 | .6 |
| Psychiatric comorbid conditions (2011) | ||
| Substance use disorder | 54,895 | 15.3 |
| Depression | 119,738 | 33.5 |
| Anxiety | 55,468 | 15.5 |
| Deliberate self-harm | 2,796 | .8 |
| General medical comorbid conditions (2011) | ||
| Diabetes | 75,833 | 21.2 |
| Serious cardiovascular disease | 126,636 | 35.4 |
| Hyperlipidemia | 86,153 | 24.1 |
| Financing arrangement | ||
| Fee for service | 246,640 | 68.9 |
| Managed care | 109,017 | 30.5 |
| Otherc | 2,257 | .6 |
| Outpatient visits for schizophrenia | ||
| 0–9 | 217,252 | 60.7 |
| 10–29 | 64,774 | 18.1 |
| 30–49 | 24,852 | 6.9 |
| ≥50 | 51,036 | 14.3 |
| Mental health emergency department visit (2011) | 92,552 | 25.9 |
| Inpatient admission for schizophrenia (N visits) | ||
| 0 | 288,876 | 80.7 |
| 1 | 34,453 | 9.6 |
| 2 | 15,819 | 4.4 |
| 3 | 6,297 | 1.8 |
| ≥4 | 12,469 | 3.5 |
| N of psychotropic medication types taken per year | ||
| 1 | 107,538 | 30.1 |
| 2 | 137,178 | 38.3 |
| 3 | 87,440 | 24.4 |
| 4 | 24,420 | 6.8 |
| 5 | 1,338 | .4 |
| Psychotropic medication typed | ||
| Any antipsychotic | 306,103 | 85.5 |
| LAI antipsychotic | 51,427 | 14.4 |
| Clozapine | 15,679 | 4.4 |
| Antidepressant | 207,531 | 58.0 |
| Mood stabilizer | 99,758 | 27.9 |
| Benzodiazepine | 97,468 | 27.2 |
TABLE 1. Demographic and service use characteristics of a national sample of persons with schizophrenia spectrum diagnoses (N=357,914)a
Demographic, Clinical, and Service Use Predictors of Psychotropic Medication Prescriptions
Table 2 presents the results from the logistic regression models predicting filled prescriptions of psychotropic classes and formulations; models adjusted for all covariates, including state. The most notable differences were observed for race-ethnicity and sex. Compared with prescriptions for non-Hispanic Whites, any antipsychotic and LAI prescriptions were more likely to be filled for non-Hispanic Blacks (adjusted OR [AOR]=1.16 for antipsychotic and AOR=1.39 for LAI), Hispanics (AOR=1.10 for antipsychotic and AOR=1.26 for LAI), and Native Hawaiians or other Pacific Islanders (AOR=1.55 for antipsychotic and AOR=1.26 for LAI). However, non-Hispanic Whites were more likely than all other racial-ethnic groups to fill prescriptions of clozapine (AOR range 1.17–1.60), antidepressants (AOR range 1.05–1.40), benzodiazepines (AOR range 1.37–1.49), and mood stabilizers (AOR range 1.26–1.31). Compared with men, women were less likely to fill prescriptions for any antipsychotic medications (AOR=0.81 for antipsychotic, AOR=0.78 for LAI, and AOR=0.82 for clozapine) but were more likely to fill prescriptions for antidepressants (AOR=1.47) and benzodiazepines (AOR=1.38) (Table 2).
| Antipsychotic medications | LAI | Clozapine | Antidepressants | Benzodiazepines | Mood stabilizers | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic characteristic | AOR | 99% CIb | AOR | 99% CIb | AOR | 99% CIb | AOR | 99% CIb | AOR | 99% CIb | AOR | 99% CIb |
| Age (continuous) | .99* | .99–.99 | .99* | .99–.99 | .98* | .98–.98 | 1.01* | 1.00–1.01 | 1.01* | 1.01–1.01 | .98* | .98–.98 |
| Women (reference: men) | .81* | .79–.83 | .78* | .76–.80 | .82* | .78–.86 | 1.47* | 1.44–1.50 | 1.38* | 1.35–1.41 | 1.00 | .98–1.02 |
| Race-ethnicity (reference: White, non-Hispanic) | ||||||||||||
| Black, non-Hispanic | 1.16* | 1.12–1.19 | 1.39* | 1.35–1.44 | .40* | .38–.42 | .71* | .69–.73 | .51* | .49–.52 | .69* | .67–.71 |
| Asian | 1.26* | 1.13–1.41 | 1.10 | .99–1.23 | .83* | .72–.96 | .60* | .56–.65 | .63* | .58–.69 | .72* | .66–.78 |
| Hispanic | 1.10* | 1.03–1.17 | 1.26* | 1.19–1.34 | .63* | .57–.69 | .95* | .92–.99 | 1.00 | .95–1.04 | .74* | .71–.78 |
| Native Hawaiian or other Pacific Islander | 1.55* | 1.30–1.84 | 1.26* | 1.11–1.44 | .80* | .66–.97 | .69* | .63–.75 | .63* | .57–.70 | .72* | .65–.80 |
| Schizophrenia subtype (reference: schizophrenia) | ||||||||||||
| Schizoaffective | 1.00 | .98–1.03 | .77* | .75–.80 | .91* | .87–.96 | 1.35* | 1.32–1.38 | 1.14* | 1.11–1.16 | 1.60* | 1.57–1.64 |
| Schizophreniform | 1.15 | .96–1.37 | .91 | .76–1.09 | .77 | .56–1.07 | .93 | .82–1.05 | 1.04 | .90–1.20 | 1.00 | .87–1.15 |
| Psychiatric comorbid condition | ||||||||||||
| Substance use disorder (reference: none) | .82* | .79–.85 | .83* | .80–.87 | .45* | .41–.50 | 1.41* | 1.37–1.45 | 1.09* | 1.06–1.13 | .88* | .86–.91 |
| Depression (reference: none) | .72* | .70–.74 | .65* | .63–.67 | .56* | .53–.60 | 2.06* | 2.01–2.10 | 1.17* | 1.14–1.20 | .92* | .90–.94 |
| Anxiety (reference: none) | .69* | .67–.72 | .66* | .63–.69 | .65* | .60–.70 | 1.41* | 1.37–1.45 | 2.33* | 2.27–2.40 | .88* | .86–.91 |
| Deliberate self-harm (reference: none) | 1.03 | .89–1.19 | 1.02 | .88–1.19 | .92 | .66–1.29 | 1.46* | 1.29–1.66 | 1.07 | .95–1.19 | 1.05 | .94–1.17 |
| Comorbid general medical condition | ||||||||||||
| Diabetes (reference: none) | 1.08* | 1.04–1.12 | 1.06* | 1.03–1.10 | 1.48* | 1.40–1.57 | 1.10* | 1.07–1.13 | 1.08* | 1.05–1.11 | 1.12* | 1.09–1.15 |
| Serious cardiovascular disease (reference: none) | .86* | .83–.89 | .84* | .81–.86 | .98* | .93–1.03 | 1.19* | 1.16–1.21 | 1.30* | 1.27–1.33 | 1.06* | 1.04–1.09 |
| Hyperlipidemia (reference: none) | 1.13* | 1.09–1.17 | .91* | .88–.94 | 1.44* | 1.37–1.52 | 1.07* | 1.05–1.10 | 1.04* | 1.01–1.07 | 1.06* | 1.03–1.08 |
| Financing arrangement (reference: FFS)c | ||||||||||||
| Managed care | .46* | .44–.48 | .81* | .77–.84 | .77* | .72–.83 | 1.24* | 1.20–1.27 | .99 | .96–1.02 | .89* | .86–.92 |
| Other | .23* | .20–.26 | .61* | .50–.74 | .16* | .08–.31 | .24* | .21–.27 | .19* | .14–.24 | .20* | .16–.25 |
| Psychiatric service use | ||||||||||||
| Outpatient schizophrenia visits (reference: 0–9) | ||||||||||||
| 10–29 | 1.94* | 1.86–2.02 | 2.65* | 2.55–2.74 | 2.36* | 2.22–2.51 | .78* | .76–.80 | .86* | .84–.89 | .89* | .86–.92 |
| 30–49 | 2.27 | 2.13–2.42 | 4.25* | 4.06–4.45 | 3.10* | 2.86–3.36 | .75* | .72–.78 | .83* | .79–.87 | .97 | .93–1.01 |
| ≥50 | 2.75 | 2.61–2.88 | 5.32* | 5.13–5.52 | 4.88* | 4.59–5.18 | .73* | .71–.75 | .82* | .79–.85 | 1.15* | 1.12–1.19 |
| Mental health ED visit (reference: none) | 1.18* | 1.14–1.22 | 1.44* | 1.39–1.49 | .77* | .72–.82 | .95* | .92–.97 | 1.12* | 1.09–1.15 | 1.36* | 1.32–1.40 |
| Inpatient schizophrenia admission visit (reference: 0) | ||||||||||||
| 1 | 1.13* | 1.08–1.19 | 1.36* | 1.30–1.43 | .92 | .84–1.00 | .91* | .88–.95 | 1.04* | 1.00–1.08 | 1.30* | 1.26–1.35 |
| 2 | 1.32* | 1.23–1.42 | 1.58* | 1.48–1.69 | 1.01 | .90–1.15 | .83* | .79–.87 | 1.04 | .99–1.10 | 1.45* | 1.38–1.52 |
| 3 | 1.53* | 1.36–1.71 | 1.80* | 1.64–1.98 | 1.12 | .93–1.34 | .83* | .77–.90 | 1.01 | .93–1.09 | 1.71* | 1.59–1.84 |
| ≥4 | 1.51* | 1.39–1.64 | 1.80* | 1.67–1.94 | 1.22* | 1.06–1.41 | .90* | .85–.96 | 1.08* | 1.01–1.15 | 1.75* | 1.65–1.85 |
TABLE 2. Likelihood of prescription of psychotropic medications for persons with schizophrenia spectrum diagnosesa
Compared with patients with other schizophrenia spectrum disorders, patients with schizoaffective disorder were less likely to fill prescriptions of LAIs or clozapine but more likely to fill prescriptions for antidepressants, benzodiazepines, and mood stabilizers. Those with comorbid substance use disorder, depression, or anxiety were less likely to fill prescriptions for antipsychotic medications and mood stabilizers but more likely to fill prescriptions for antidepressants (AOR range 1.41–2.06) and benzodiazepines (AOR range 1.09–2.33) than were patients without these comorbid conditions. Deliberate self-harm was associated with increased odds of antidepressant prescriptions (AOR=1.46) (Table 2) but not with prescriptions of other medication classes. All general medical comorbid conditions were associated with increased likelihood of benzodiazepine and mood stabilizer prescription. Those under managed care were more likely to be prescribed antidepressants (AOR=1.24) and less likely to be prescribed all other medication types than those under FFS financing arrangements.
State Variation in the Prescription of Psychotropic Medications
Adjusted proportions of patients in each state who filled prescriptions for LAI medications, clozapine, and benzodiazepines are shown in Figures 1–3, and information for each psychotropic medication is provided in the online supplement. States significantly differed in prescribing for each of the medication types (LAIs, χ2=4,893.0, df=47, p<0.001; clozapine, χ2=2,709.1, df=47, p<0.001; antidepressants, χ2=4,092.1, df=47, p<0.001; benzodiazepines, χ2=8,186.2, df=47, p<0.001; and mood stabilizers, χ2=858.5, df=47, p<0.001).
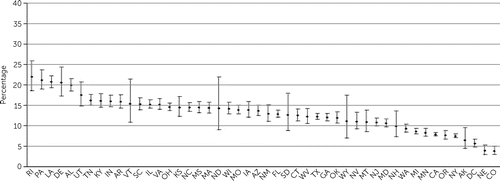
FIGURE 1. Adjusted proportions of long-acting injectable antipsychotic prescription, by U.S. statea
aDifference among states (error bars indicate 99% confidence intervals): χ2=4,892.99, df=47, p<0.001. Standard two-letter state abbreviations are used on the x-axis.
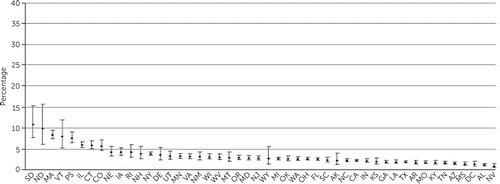
FIGURE 2. Adjusted proportions of clozapine prescription, by U.S. statea
aDifference among states (error bars indicate 99% confidence intervals): χ2=2,709.06, df=47, p<0.001. Standard two-letter state abbreviations are used on the x-axis.
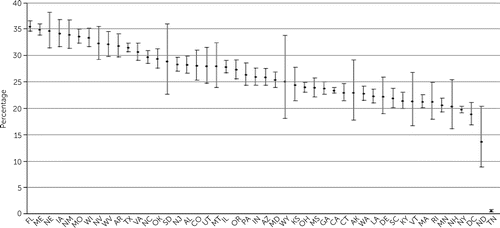
FIGURE 3. Adjusted proportions of benzodiazepine prescription, by U.S. statea
aDifference among states (error bars indicate 99% confidence intervals): χ2=8,186.17, df=47, p<0.001. Standard two-letter state abbreviations are used on the x-axis.
Proportions of LAI prescriptions ranged from 4% (in Colorado) to 22% (in Rhode Island) (Figure 1), an adjusted fivefold difference and interquartile range (IQR) of 4%. For clozapine, some states (North Dakota, South Dakota, and Vermont) with few patients were outliers, but prescriptions in most states ranged from 1% (in Nevada) to 11% (in South Dakota), representing an adjusted 10-fold difference and IQR of 2% (Figure 2). Adjusted proportions for antidepressants ranged from 47% (in Vermont) to 75% (in Missouri), representing a 55% difference and IQR of 8%. Two states (Tennessee and North Dakota) were outliers in benzodiazepine prescriptions, but prescription in the other states ranged from about 20% to 35%, representing a 75% difference and IQR of 8% (Figure 3). Mood stabilizer prescriptions ranged from 19% (in Montana) to 33% (in Pennsylvania), representing a 73% difference and an IQR of 5%.
Post Hoc Analyses
As a result of the adjustment for all factors used in these analyses to identify whether heterogeneity in prescribing patterns persisted, identification of statewide differences in these factors and their possible implications was limited. To probe whether patient race-ethnicity or other characteristics contributed to interstate variation in prescribing patterns, we partitioned states by tertiles of LAI medication and clozapine prescriptions (i.e., one record per state for highest, middle, and lowest levels of LAI and clozapine prescriptions). We then examined the composition of characteristics of patients in these state-level tertiles of LAI and clozapine prescriptions (see table in the online supplement).
Results from these analyses revealed that states in the highest compared with the lowest tertile of LAI medication prescription had higher percentages of non-Hispanic Whites (43.8% [N=34,831 of 79,442] vs. 36.6% [N=59,296 of 161,860]) and non-Hispanic Blacks (43.5% [N=34,542 of 79,442] vs. 31.7% [N=51,351 of 161,860]) and lower percentages of Hispanics (2.8% [N=2,216 of 79,442] vs. 15.9% [N=25,670 of 161,860]). States in the highest compared with the lowest tertile of clozapine prescription had lower percentages of non-Hispanic Blacks (32.0% [N=26,777 of 83,683] vs. 41.9% [N=42,594 of 101,734]) and higher percentages of Hispanics (18.6% [N=15,547 of 83,683] vs. 7.1% [N=7,180 of 101,734]).
As a measure of access to psychiatrists, we calculated the number of psychiatrists per capita with Census Bureau data from 2012 for the states in our study. States with higher rates of clozapine prescription (Colorado, Connecticut, Delaware, Illinois, Iowa, Massachusetts, Montana, Nebraska, New Hampshire, New York, North Dakota, Pennsylvania, Rhode Island, South Dakota, Utah, Vermont) had more psychiatrists per capita (11 psychiatrists per 100,000 residents) than those with lower rates (Alabama, Alaska, Arizona, Arkansas, District of Columbia, Georgia, Indiana, Kansas, Kentucky, Louisiana, Mississippi, Missouri, Nevada, South Carolina, Tennessee, Texas) (five psychiatrists per 100,000 residents).
Discussion
The results of this study indicated variation across states in prescription patterns for psychotropic medications to treat people diagnosed as having schizophrenia. The most dramatic interstate differences were in prescription of clozapine and LAIs, which have distinctive roles in medication management—clozapine has efficacy in managing treatment-resistant schizophrenia and reducing suicidal behaviors and has low rates of prescription by clinicians (15, 32–39), and LAI medications address nonadherence, but they require clinician administration and pose risks for coercion (5, 40). Significant variation across states held for all the psychotropic medications studied, even after the analysis was adjusted for several relevant covariates. This national analysis expands previous investigations of specific city (41), state (42), and regional (43) variations in prescriptions for individuals with schizophrenia by including a range of psychotropic medications.
Identification of individual-level predictors of psychotropic medication prescription aids our understanding of state-level variation in prescribing practices. Non-Hispanic Whites were more likely to fill clozapine and less likely to fill LAI prescriptions than were non-Hispanic Blacks, Hispanics, and people of all other race-ethnicities. These findings were based on a national data set and are consistent with those from previous reports (15, 20, 21), further highlighting the need to understand drivers of disparities and variation in state-level prescription of these medications. One hypothesis is that availability of providers that prescribe clozapine, and that offer regular monitoring of neutrophil counts required for clozapine maintenance, may be unevenly distributed in states where significant numbers of people from racial-ethnic minority groups receive services. These providers may recognize treatment-resistant schizophrenia and other indications for clozapine better among non-Hispanic Whites than among other racial-ethnic groups. Conversely, the divergent patterns of LAI medication prescription may be due to interstate differences in the type of insurance, clinical and demographic factors of patients or psychiatrist demographic characteristics (44). For example, some providers may be better at recognizing indications for LAIs, including medication nonadherence or risk factors such as substance use disorder, poor insight, or risk for relapse associated with aggression, violence, or self-harm (45), among non-Hispanic Blacks, Hispanics, and other non-White groups. Interest in addressing medication nonadherence (44) and awareness that LAIs may be stigmatizing from the patient’s perspective (46) may influence decision making of clinicians qualified to administer LAI medications.
That racial-ethnic minority groups were consistently less likely than non-Hispanic Whites to fill prescriptions for antidepressants and mood stabilizers may have reflected receipt of care in settings with fewer resources and receipt of narrower evaluations leading to poorer recognition of mood and anxiety problems. State regulation of benzodiazepines may have affected their use—Tennessee’s Medicaid program had strict limits on benzodiazepine prescriptions (47). Concern regarding benzodiazepine abuse liability that has resulted in changes in labeling by the U.S. Food and Drug Administration (48) may be associated with disproportionately lower numbers of prescriptions of these medications to patients from racial-ethnic minority groups compared with non-Hispanic Whites (49). These prescription patterns raise questions about the equity and quality of care provided to different racial-ethnic groups, and if rooted in implicit biases or systemic racism, disparities in prescription must be addressed to ensure equal access to evidence-based treatments for everyone.
The variability in prescribing patterns of psychotropic medications across states should be viewed in the context of several limitations. Because this was an observational study, we could not account for unmeasured variation in several comparisons. The data reflected filled prescriptions, not medication ingestion. We were also limited by using clinical rather than research diagnoses, although clinical diagnoses are appropriate for describing real-world community practice patterns. This study used the latest year that included data from almost all states; practice patterns may have changed since 2012, including expansion of Medicaid coverage through the Affordable Care Act. Our analysis was limited to individuals diagnosed as having schizophrenia in 2011 and to 2012 data from these individuals; therefore, we could not account for the length of schizophrenia diagnosis that could influence initiation and use of LAI medications after multiple oral antipsychotic medications had failed to improve patients’ conditions. The data did not include patients dually eligible for Medicaid and Medicare, those privately insured or insured by the U.S. Department of Veterans Affairs, or uninsured adults. Previous studies have found that type of practitioner (e.g., psychiatrist vs. general practitioner) is related to selection of psychotropic medications, but Medicaid data do not include a consistent provider type variable for prescriptions (50).
Some of the variation in psychotropic prescribing patterns might be explained by state preferred drug lists (PDLs). Pennsylvania, for example, which had the fifth highest adjusted odds of LAI prescribing, has no current prior authorization requirements for LAI medications (51). By contrast, Colorado, which had the lowest adjusted odds of LAI prescription, requires prior authorization for all LAIs (52). Without access to 2012 preferred drug lists from each of the states, however, a formal analysis of the contribution of state PDLs to prescribing practices for people with schizophrenia is beyond the scope of our analysis.
Conclusions
Our results indicate significant variation across states and among racial-ethnic groups in prescription patterns of six types of psychotropic medications, even after we had adjusted for multiple patient factors. Variations in state policies and differences in psychiatrist training and prescribing behaviors may have contributed to these variations. Psychiatric training that is culturally sensitive and seeks to minimize disparities by race or ethnicity and that requires competency in the prescription of clozapine and LAI antipsychotic medications may reduce variation. The use of standardized PDLs that have fewer LAI medications requiring prior authorization and that list more LAIs and oral antipsychotic medications may be a concrete way to improve prescribing patterns. A better understanding of the causes of wide variation in LAI and clozapine prescriptions is needed to improve access to these important treatment options.
1 : Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 2017; 74:675–684Crossref, Medline, Google Scholar
2 : Psychotropic medication use among adults with schizophrenia and schizoaffective disorder in the United States. Psychiatr Serv 2018; 69:605–608Link, Google Scholar
3 : The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull 2010; 36:94–103Crossref, Medline, Google Scholar
4 : Adherence assessments and the use of depot antipsychotics in patients with schizophrenia. J Clin Psychiatry 2001; 62:545–551Crossref, Medline, Google Scholar
5 : State and demographic variation in use of depot antipsychotics by Medicaid beneficiaries with schizophrenia. Psychiatr Serv 2014; 65:121–124Link, Google Scholar
6 : Ethical use of long-acting medications in the treatment of severe and persistent mental illnesses. Compr Psychiatry 2004; 45:161–167Crossref, Medline, Google Scholar
7 : Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry 2019; 76:508–515Crossref, Medline, Google Scholar
8 : Lithium for schizophrenia. Cochrane Database Syst Rev 2015; 10:CD003834Google Scholar
9 : Valproate for schizophrenia. Cochrane Database Syst Rev 2008; 3:CD004028Google Scholar
10 : Carbamazepine for schizophrenia. Cochrane Database Syst Rev 2014; 5:CD001258Google Scholar
11 : Lamotrigine for schizophrenia. Cochrane Database Syst Rev 2006; 4:CD005962Google Scholar
12 : Benzodiazepines for schizophrenia. Cochrane Database Syst Rev 2012; 11:CD006391Medline, Google Scholar
13 : Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med 1982; 16:811–824Crossref, Medline, Google Scholar
14 : Variation in ECT use in the United States. Am J Psychiatry 1995; 152:869–875Link, Google Scholar
15 : Guideline Watch (Sept 2009): Practice Guideline for the Treatment of Patients With Schizophrenia. APA Practice Guidelines. Washington, DC, American Psychiatric Association, 2009. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia-watch.pdfGoogle Scholar
16 : Clozapine for schizophrenia: state variation in evidence-based practice. Psychiatr Serv 2016; 67:152Link, Google Scholar
17 : Use of Pooled State Administrative Data for Mental Health Services Research. Adm Policy Ment Health 2016; 43:67–78Crossref, Medline, Google Scholar
18 : Antipsychotic prescribing practices in Connecticut’s public mental health system: rates of changing medications and prescribing styles. Schizophr Bull 2002; 28:17–29Crossref, Medline, Google Scholar
19 : Antipsychotic prescribing practices in the Veterans Healthcare Administration–New York metropolitan region. Schizophr Bull 2002; 28:31–42Crossref, Medline, Google Scholar
20 : Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv 2017; 68:579–586Link, Google Scholar
21 : The effect of race-ethnicity on the comparative effectiveness of clozapine among Medicaid beneficiaries. Psychiatr Serv 2013; 64:230–237Link, Google Scholar
22 : National trends in psychotropic medication polypharmacy in office-based psychiatry. Arch Gen Psychiatry 2010; 67:26–36Crossref, Medline, Google Scholar
23 : Polypharmacy for schizophrenia. Curr Opin Psychiatry 2013; 26:208–213Crossref, Medline, Google Scholar
24 : Accuracy of diagnoses of schizophrenia in Medicaid claims. Hosp Community Psychiatry 1992; 43:69–71Abstract, Google Scholar
25
26 : Metabolic syndrome associated with schizophrenia and atypical antipsychotics. Curr Diab Rep 2010; 10:209–216Crossref, Medline, Google Scholar
27 : Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 2009; 24:412–424Crossref, Medline, Google Scholar
28 : The risk of head injuries associated with antipsychotic use among persons with Alzheimer’s disease. J Am Geriatr Soc 2020; 68:595–602Crossref, Medline, Google Scholar
29 : Drug-induced dyskinesia, part 2: treatment of tardive dyskinesia. Drugs 2016; 76:779–787Crossref, Medline, Google Scholar
30 : Psychiatric treatment of persons with HIV/AIDS: an HIV-psychiatry consensus survey of current practices. Psychosomatics 2010; 51:480–488Crossref, Medline, Google Scholar
31 SAS 9.4. Cary, NC, SAS Institute, 2013Google Scholar
32 : Evaluating the effect of the changes in FDA guidelines for clozapine monitoring. J Clin Psychiatry 2017; 78:e933–e939Crossref, Medline, Google Scholar
33 : An initiative to improve clozapine prescribing in New York State. Psychiatr Serv 2016; 67:369–371Link, Google Scholar
34 : Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
35 : Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003; 60:82–91Crossref, Medline, Google Scholar
36 : The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull 2010; 36:71–93Crossref, Medline, Google Scholar
37 : Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry 2017; 74:686–693Crossref, Medline, Google Scholar
38 : Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry 2004; 161(suppl 2):1–56Link, Google Scholar
39 : The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 2020; 177:868–872Link, Google Scholar
40 : Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull 2018; 44:603–619Crossref, Medline, Google Scholar
41 : Racial disparities in the use of second-generation antipsychotics for the treatment of schizophrenia. Psychiatr Serv 2006; 57:133–136Link, Google Scholar
42 : Racial disparities in antipsychotic prescription patterns for patients with schizophrenia. Am J Psychiatry 2002; 159:567–572Link, Google Scholar
43 : Racial differences in antipsychotic use: claims database analysis of Medicaid-insured patients with schizophrenia. Ann Clin Psychiatry 2015; 27:242–252Medline, Google Scholar
44 : Use of depot antipsychotic medications for medication nonadherence in schizophrenia. Schizophr Bull 2008; 34:995–1001Crossref, Medline, Google Scholar
45 : The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry 2016; 77(suppl 3):1–24Crossref, Medline, Google Scholar
46 : Psychiatrists’ use, knowledge and attitudes to first- and second-generation antipsychotic long-acting injections: comparisons over 5 years. J Psychopharmacol 2010; 24:1473–1482Crossref, Medline, Google Scholar
47 Medicaid Coverage of Benzodiazepines and Barbiturates. Alexandria, VA, National Mental Health Association, n.d. https://www.mhanational.org/sites/default/files/MedicareExcludedDrugChart.pdfGoogle Scholar
48 FDA Requiring Labeling Changes for Benzodiazepines: Boxed Warning to Be Updated to Include Abuse, Addiction and Other Serious Risks. Silver Spring, MD, US Food and Drug Administration, n.d. https://www.fda.gov/news-events/press-announcements/fda-requiring-labeling-changes-benzodiazepines. Accessed Dec 11, 2020Google Scholar
49 : Examining racial/ethnic differences in patterns of benzodiazepine prescription and misuse. Drug Alcohol Depend 2018; 187:29–34Crossref, Medline, Google Scholar
50 : Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA 1998; 279:526–531Crossref, Medline, Google Scholar
51 Statewide Preferred Drug List (PDL). Harrisburg, PA, Pennsylvania Department of Human Services, n.d. https://papdl.com/sites/default/files/ghs-files/Penn%20Statewide%20PDL%2001.05.21.pdfGoogle Scholar
52 Preferred Drug List (PDL). Denver, Colorado Department of Health Care Policy & Financing. https://hcpf.colorado.gov/pharmacy-resources. Accessed Sep 7, 2021Google Scholar



