Assessing the Long-Term Effectiveness of a Behavioral Health Home for Adults With Bipolar and Psychotic Disorders
Abstract
Objective:
This study aimed to examine the impact of a behavioral health home (BHH) to better understand its potential to improve health for individuals with serious mental illness.
Methods:
Propensity score–weighted interrupted time series analysis was used to estimate service utilization and chronic disease management through 3.5 years after BHH implementation and to compre BHH enrollees (N=413) with other patients with serious mental illness in the same health system (N=1,929).
Results:
Relative to control group members, BHH patients had an immediate increase in primary care visits (+0.18 visits/month), which remained higher throughout follow-up, and an immediate decrease in emergency department visits (–0.031 visits/month). Behavioral health outpatient visits, which were increasing for BHH participants before implementation, began decreasing postimplementation; this decrease (–0.016 visits/month) was significantly larger than for the control group. Inpatient and outpatient visits for general medical health were decreasing over time for both groups before implementation but decreased more slowly for BHH patients postimplementation. Although behavioral health inpatient visits decreased for both groups around the start of the BHH program and remained lower, this initial drop was larger for the non-BHH group. BHH participation was associated with decreases in hemoglobin A1c values but no shift in low-density lipoprotein cholesterol values.
Conclusions:
The results reflect the challenges of improving health for patients with serious mental illness, even as access to primary care is increased. Further study is needed about which complex interventions inside and outside of the health care system can help offset the 20- to 30-year mortality gap faced by this population.
Highlights
Enrollment in a behavioral health home (BHH) that integrated primary care into a specialty outpatient behavioral health setting was associated with an immediate increase in primary care visits, a decrease in emergency department use, and a decrease in behavioral health outpatient visits over 3.5 years of follow-up.
BHH participants had improved hemoglobin A1c but no change in low-density lipoprotein cholesterol values, and participation was associated with higher rates of metabolic monitoring during the entire observation period.
Future research should focus on how BHH programs can support behavior changes that improve cardiovascular health.
Adults with serious mental illness face a 20- to 30-year mortality gap (1–3) driven by poor access to high-quality primary care (4–10), metabolic side effects of antipsychotic medications (11–13), and other factors (14–16) that increase risk for cardiometabolic disease (17, 18). Behavioral health homes (BHHs) help mitigate these mortality disparities by integrating primary care and wellness services into behavioral health clinics and enhancing care coordination via care management (1–3, 16, 19–27).
A 2018 systematic review found that BHH models improved cardiometabolic screening but that health impacts were limited (28). Some studies reported improvement in general medical health (GMH) outcomes (23), and others found improved GMH process measures but not outcomes (24, 29). Maryland’s Medicaid Medical Home model showed reduction in emergency department (ED) visits for GMH (30), but the study had small effect sizes and showed no observed effect on inpatient admissions. Because early BHH demonstrations were promising (9, 23, 31–33), adoption of these models has increased. As clinic and hospital systems adopt value-based payment models and shift to population health approaches for designing clinical care, interest has increased in understanding BHH’s impacts on health and service utilization.
Few longitudinal evaluations have been conducted on BHH implementation. Many studies also lack a comparison group or emphasize vulnerable subpopulations (23). To close these literature gaps and understand the BHH’s potential to improve health for individuals with serious mental illness, we analyzed longer-term (3.5 years) impacts on health care utilization among all participants and vulnerable subgroups as well as metabolic screening and cardiometabolic rates. A prior 1-year evaluation found that BHH participants had improvements in hemoglobin A1c (HbA1c) screening rates and marginal improvement in lipid monitoring rates and had fewer psychiatric inpatient and total ED visits (34).
Methods
Setting and Intervention
A BHH was established in September 2015 at a community-based behavioral health clinic in an urban, safety-net health system caring for about 140,000 patients annually. Patients with primary psychotic or bipolar disorders (plus any antipsychotic prescription) receiving care at the clinic were automatically enrolled in the BHH on September 1, 2015. Referrals within the health system were accepted on the basis of set criteria for psychiatric diagnosis, medical risk, and care coordination needs. The BHH sought to increase access to primary care through intensive referrals and service colocation, but neither primary care visits, nor any other kind of visit for GMH care, were required for participants. (For key BHH components, see part 1 in an online supplement to this article.)
Study Period and Sample
The BHH implementation start date was September 1, 2015; our observation period included service encounters 1 year preintervention (September 1, 2014–August 31, 2015) and 3.5 years postintervention (September 1, 2015–February 28, 2019).
Our study sample consisted of all patients receiving care in the health system during the observation period before October 31, 2015, with diagnoses of either primary psychotic disorders or bipolar disorder accompanied by an antipsychotic medication. Data from patients whose BHH enrollment was documented in the first 2 months of enrollment were included in this analysis (N=413). Those enrolled after this date (N=304) were included in a sensitivity analysis (see part 2 in the online supplement). Our control group consisted of patients from the same health system diagnosed as having serious mental illness who never enrolled in the BHH and who had at least one specialty behavioral health visit in the pre- and postintervention periods.
Electronic health record data were accessed for individual-level demographic characteristics (age, sex, race, language spoken at home, marital status), medical information (diagnosis, prescriptions, health insurance type), and health encounter information (date and type, lab values). Area-level variables (percentage foreign born, percentage living in poverty, percentage of female-headed households, and percentage with less than a high school education) were sourced from census data on individual zip codes or were imputed for patients with missing zip codes by using the “mi impute” command in Stata (35).
Statistical Analysis
We employed multigroup interrupted time series analysis (ITSA), which compares pre- and postintervention trends from longitudinal data between the treatment sample and a representative control sample. The control sample adjusted for exogenous shocks that could influence outcomes of interest.
To adjust for differences between samples, we used inverse propensity weighting (IPW) to weight individuals from the control sample based on how they resembled the BHH sample (36). Propensity scores were calculated by using individual and area-level characteristics. We did not include service use variables in the propensity score to avoid potential bias due to regression to the mean (37). Control group members with scores lower than the lowest value in the BHH sample were dropped. To estimate average treatment effect on the treated sample, standardized mortality ratio weights (38) were generated from scores and applied to individual observations for both BHH patients and control group members.
Autocorrelation was assessed with the Cumby-Huizinga test. When first-order autocorrelation was detected, Newey-West standard errors were replaced with Prais-Winsten standard errors. Newey-West standard errors were used to account for heteroskedasticity and autocorrelation. Monthly indicators were added to our models to control for seasonal variation. All analyses were conducted in StataMP, version 15.1 (39).
Primary and Secondary Outcomes
Our ITSA followed Linden (40). We applied individual propensity scores to weight the number of monthly service encounters for each individual. The sample average of these values (the sum of individual weighted values divided by the sample size) was the dependent variable in the ITSA regression model.
We considered six visit types as dependent variables: primary care, ED, GMH inpatient, GMH outpatient (i.e., other types of specialty GMH care), behavioral health inpatient, and behavioral health outpatient. Our primary outcomes of interest were, first, the difference in predicted monthly visits immediately after the intervention start date (“level shift”). Level shifts represent abrupt changes in patient behavior that occurred as soon as the intervention began. Second, we considered how the rate of monthly visit counts changed (i.e., whether counts were falling or rising, and by how much) over the 3.5-year follow-up period compared with the preintervention period (“trend shift”). A negative trend shift would occur, for instance, if utilization declined at a faster rate than in the preintervention period. Level shifts represent immediate intervention impacts, whereas trend shifts capture the intervention’s impact on outcome trajectories over time. Our ITSA model analyzed primary outcomes through a difference-in-difference (DID) framework, comparing shifts between BHH enrollees versus control group members.
Secondary outcomes included the average individual-level change in cardiometabolic measures between the pre- and postintervention period for the two samples. We conducted this analysis for all individuals with at least one value recorded in the pre- and postintervention period and, as a sensitivity analysis, among only those who had a categorically high value recorded in the preintervention period (greater than 5.6% for HbA1c and greater than 190 mg/dL for low-density lipoprotein [LDL] cholesterol).
Exploratory Subgroup Analyses
Subgroup analyses assessed how the intervention’s impact on the assessed variables varied by race-ethnicity and language spoken at home, both between the BHH and control groups and within the BHH group (no IPW applied). This study was approved by the institutional review board of the Cambridge Health Alliance.
Results
Study Sample
The demographic and clinical characteristics of 413 patients who enrolled in the BHH between September 1 and October 31, 2015, were compared with those of 1,929 control group members. Table 1 shows differences by sample before IPW. More BHH patients than control group members had primary psychotic diagnoses (86% vs. 51%), and fewer had bipolar diagnoses (31% vs. 61%). BHH patients were also more likely to identify as non-Hispanic Black (23% vs. 16%) and more likely to be a native English speaker (91% vs. 80%).
| Control group | Behavioral health home | |||||
|---|---|---|---|---|---|---|
| (N=1,929) | (N=413) | |||||
| Characteristic | N | % | N | % | p | |
| Age (M±SD) | 50.03±.39 | 50.74±.70 | .42 | |||
| Female | 1,036 | 53.71 | 186 | 45.04 | <.01 | |
| Native English speaker | 1,547 | 80.20 | 374 | 90.56 | <.01 | |
| Diabetes | 344 | 17.83 | 78 | 18.89 | .62 | |
| Primary psychotic disorder | 979 | 50.75 | 355 | 85.96 | <.01 | |
| Bipolar disorder | 1,182 | 61.28 | 128 | 30.99 | <.01 | |
| Race-ethnicity | ||||||
| Asian | 70 | 3.63 | 18 | 4.36 | .48 | |
| Hispanic | 168 | 8.71 | 6 | 1.94 | <.01 | |
| Non-Hispanic Black | 302 | 15.66 | 95 | 23.00 | <.01 | |
| Non-Hispanic White | 1,183 | 61.33 | 262 | 63.44 | .42 | |
| Portuguese or Brazilian | 158 | 8.19 | 20 | 4.84 | .02 | |
| Health insurance | ||||||
| Medicaid | 1,027 | 53.24 | 181 | 43.83 | <.01 | |
| Medicare | 737 | 38.21 | 209 | 50.61 | <.01 | |
| Private | 156 | 8.09 | 23 | 5.57 | .08 | |
| Self-pay | 8 | .41 | 0 | .19 | ||
| Marital status | ||||||
| Divorced | 216 | 11.20 | 33 | 7.99 | .05 | |
| Legally separated | 67 | 3.47 | 5 | 1.21 | .02 | |
| Married | 289 | 14.98 | 37 | 8.96 | <.01 | |
| Single | 1,263 | 65.47 | 329 | 79.66 | <.01 | |
| Widowed | 78 | 4.04 | 9 | 2.18 | .07 | |
| Area-level indicators (M±SD %) | ||||||
| Female-headed households | 14.62±.19 | 14.85±.37 | .60 | |||
| Less than a high school education | 13.75±.22 | 10.21±.38 | <.01 | |||
| Foreign born | 30.66±.26 | 27.97±.51 | <.01 | |||
| Living in poverty | 13.87±.15 | 13.43±.30 | .23 | |||
TABLE 1. Demographic characteristics of participants, by treatment group
After we conducted IPW, all characteristics were balanced across the two samples (Figure 1 and part 3 of the online supplement). For the primary analyses, 61 control group members were excluded because their propensity scores were outside the range of that for BHH patients (i.e., scores were not good for comparison).
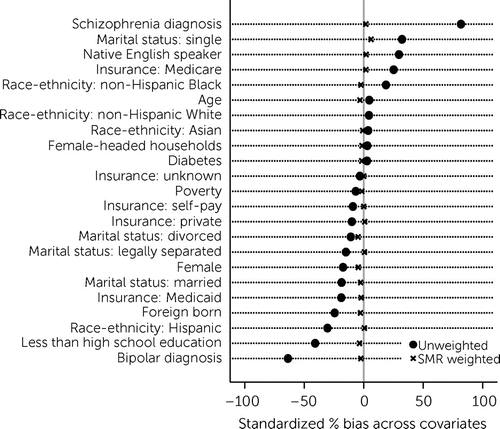
FIGURE 1. Covariate balance between the behavioral health home and control groups before and after standardized mortality ratio (SMR) propensity score weightinga
a Percentage with less than high school education, percentage foreign born, percentage living in poverty, and percentage of female-headed households were recorded at the area level on the basis of patient zip code data.
Service Utilization DID
Figure 2 displays data from the ITSA for our primary outcomes. Table 2 presents the multigroup ITSA estimates for utilization shifts between the pre- and postintervention period among the treatment and control groups. (All preintervention values are available in part 4 of the online supplement.)
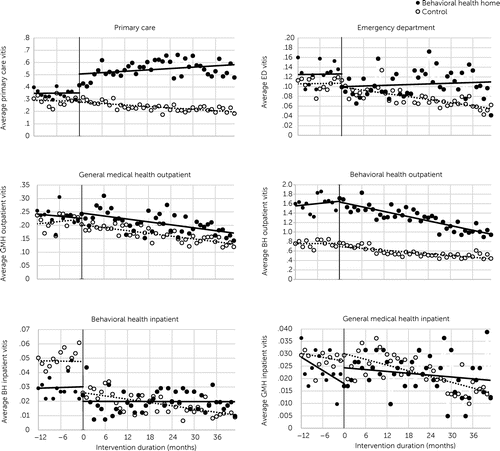
FIGURE 2. Weighted average monthly care visits, by service type and treatment groupa
a Y-axis range depends on utilization outcome to facilitate readability. The vertical line represents the beginning of the intervention period (month 0). BH, behavioral health; ED, emergency department; GMH, general medical health.
| Pre-post intervention group shifts | Difference-in-difference shifts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (for contextualization) | (primary outcomes)b | |||||||||||
| BHH level shift | BHH trend shift | Control level shift | Control trend shift | Level shift | Trend shift | |||||||
| Visit type | Value | SE | Value | SE | Value | SE | Value | SE | Value | SE | Value | SE |
| Primary care | .20** | −.03 | .008 | −.005 | .004 | −.03 | .0003 | −.004 | .18** | −.04 | .006 | −.004 |
| ED | −.03* | −.01 | −.00009 | −.002 | .003 | −.008 | .0004 | −.001 | −.03* | −.01 | −.0002 | −.002 |
| GMH inpatient | .006 | −.005 | .0007 | −.0006 | .004 | −.003 | −.00003 | −.0003 | .002 | −.003 | .0007** | −.0002 |
| GMH outpatient | .06** | −.01 | .005** | −.001 | −.01 | −.01 | −.002 | −.001 | .06** | −.01 | .004** | −.001 |
| Behavioral health inpatient | −.01 | −.008 | −.0003 | −.0009 | −.03** | −.004 | −.001** | −.0005 | .02* | −.008 | .001 | −.0008 |
| Behavioral health outpatient | −.03 | −.05 | −.03** | −.005 | −.09* | −.04 | −.009** | −.003 | .07 | −.05 | −.02** | −.004 |
TABLE 2. Main interrupted time series shifts due to BHH participationa
In the year before BHH enrollment, average primary care utilization was 0.36 visits per month (falling at rate of 0.0057 fewer visits per month) for eventual BHH enrollees and 0.29 visits per month (falling at a rate of 0.0022 fewer visits per month) for the control sample. Postimplementation, BHH patients experienced an immediate level shift of 0.20 more primary care visits per month, compared with 0.0037 more visits per month among the control sample, resulting in a level shift DID of 0.18 more primary care visits per month (p<0.01) for those enrolled in BHH. BHH enrollment was also associated with an immediate decrease in ED visits (DID level shift=–0.031, p<0.01) and increase in specialty GMH outpatient visits (DID level shift=+0.055, p<0.01). Although both BHH and non-BHH participants showed a drop in behavioral health inpatient visits after BHH program implementation, BHH participants showed a shallower drop, suggesting a relative increase (DID level shift=+0.018, p<0.05). If level shifts were applied equally across the first year (not just the first month, as estimated by our model), BHH participation would be associated with 2.16 more primary care, 0.36 fewer ED, 0.66 more GMH outpatient, and 0.036 more behavioral health inpatient visits during that year.
When considering the intervention’s impact on the trajectory of outcomes (trend shifts), the rate of change among BHH patients for behavioral health outpatient visits was 0.024 fewer during the postintervention period compared with the preintervention period; this measure was estimated at 0.0090 fewer in the control sample. Within the DID model, BHH enrollment was associated with 0.016 fewer behavioral health outpatient visits every month after the intervention began (DID trend shift, p<0.01). Although GMH inpatient and outpatient visits were decreasing over time for both groups, relative to the control group, BHH enrollment was also associated with small increases in the monthly rate of GMH inpatient visits (DID trend shift=+0.00074, p<0.01) and GMH outpatient visits (DID trend shift=+0.0044, p<0.01). If trend shifts were applied equally across the 3-year postintervention period, BHH participation would be associated with 0.19 fewer behavioral health outpatient, 0.009 more GMH inpatient, and 0.05 more GMH outpatient visits for every year of observation. Shifts not listed here were not statistically significant and are presented in Table 2.
Subgroup Analyses
When restricting the sample to non-White patients and those with English as a second language, intervention effects were generally smaller or nonsignificant. The DID level shift in primary care visits for BHH enrollees after the intervention was 0.184 in the entire sample, but this value decreased to 0.167 when the sample was restricted to non-White patients (BHH patients, N=139; control group members, N=698); no associated level shift in primary care visits was detected when the entire sample was restricted to non-English speakers (BHH patients, N=39; control group members, N=382).
Compared with non-White BHH enrollees (N=139), White enrollees (N=274) experienced minor differences only for behavioral health outpatient visits. Among BHH enrollees, native English speakers (N=374) experienced a greater immediate increase in primary care visits and fewer ED visits (both immediately and over time) than did nonnative English speakers (N=39). Results for the subgroup analyses were exploratory and should be interpreted with caution because of small sample sizes (see part 5 of the online supplement).
Cardiometabolic Measures
To assess how BHH enrollment affected cardiometabolic measures, we restricted our sample to participants who had at least one laboratory test value in the pre- and postintervention period (N=186 BHH patients and 498 control group members for HbA1c; N=174 BHH patients and 475 control group members for LDL cholesterol). When comparing average laboratory test values from the pre- and postintervention periods between BHH patients and control group members, we detected no statistically significant change in LDL levels, but BHH participation was associated with 0.29 fewer percentage points for HbA1c (p<0.05). When restricted to those with high values in the preintervention period (504 control group members and 148 BHH patients for HbA1c; 10 control group members and six BHH patients for LDL), BHH participation was associated with 0.47 fewer percentage points for HbA1c (p<0.05). Data from all 10 control group members in the subsample with high LDL values were dropped because the propensity scores of these members were too low. An exploratory analysis suggested higher cardiometabolic screening rates among BHH participants compared with the control population (see part 6 of the online supplement).
Discussion
The findings of this study suggest that BHH enrollment altered health care utilization patterns. Relative to control group members, BHH patients had an immediate increase in primary care visits, which held during the 3.5-year follow-up, and an immediate decrease in ED visits. Behavioral health outpatient visits were increasing for BHH patients before program implementation but fell postimplementation at a rate significantly faster than for control group members. GMH inpatient and outpatient visits were decreasing over time for both groups but decreasing more slowly for BHH patients postimplementation. Although behavioral health inpatient visits decreased for both groups postimplementation, this initial drop was larger for the control group. Our model indicated small immediate shifts toward increased GMH subspecialty and GMH inpatient visits among the BHH patients. Although these shifts were small and qualitatively subtle, as seen in Figure 2, they may indicate BHH participants’ increasing engagement with emergent needs. Because our models estimated the average treatment effect on those receiving treatment, results correspond to the patients who were ultimately selected into the BHH program and are generalizable to patients who resemble our patient population (i.e., those who may enroll into such programs in the future).
Our results align with two recent reviews of BHHs (28, 41), which found that most BHHs have increased utilization of primary care visits with mixed effects on other service utilization. This literature includes a randomized controlled trial of a similar BHH model (e.g., integrated nurse practitioner and care manager), for which results are more robust than in other studies. Our findings are more consistent with BHHs that share such comprehensive features; future analyses should better describe program components to facilitate cross-study comparisons (see part 1 of the online supplement).
An increase in primary care visits occurring alongside a decrease in outpatient behavioral health visits suggests that before BHH implementation, some behavioral health utilization may have addressed GMH needs that were later met by the BHH’s primary care services.
An exploratory analysis suggested that HbA1c decreased for BHH participants with higher screening rates, especially if HbA1c values were high before the intervention. Other BHH evaluations detected no such association between BHH participation and HbA1c lab values. Collectively, the results suggest that BHH programs can improve health outcomes for those with serious mental illness, but only if these programs move beyond ensuring timely screening to providing targeted interventions that mitigate the risk that newly identified risk factors present. Failing to target the whole person will achieve minimal success (28). Evaluating the features of the BHH in this analysis that led to improved cardiometabolic values would be a fruitful research question.
Ours is one of few studies to consider heterogeneity in BHH outcomes by patients’ race-ethnicity and language. New initiatives should acknowledge health equity by building in robust data collection to understand disparities in enrollment and engagement. Mixed-methods studies that address unmet needs for patients with serious mental illness from diverse backgrounds are encouraged.
There is growing interest in developing value-based payment models that facilitate the integration of general medical and behavioral health care (42, 43), which have been shown to be cost-effective (44–46). The results of this study suggest that BHHs may increase primary care visit utilization without increasing visits to specialty behavioral health outpatient care. Although we did not consider economic outcomes, future studies using claims data may consider the long-term economic consequences of BHH programs. This is critical for decision makers who seek to improve care coordination in the context of value-based payment models.
Study Strengths
The follow-up period of our analysis (3.5 years) was longer than other analyses on BHHs and allowed us to distinguish between immediate and long-term effects of the intervention (level vs. trend shifts). In relation to a prior analysis of this BHH based on a 1-year follow-up (34), the present analysis confirmed that the increase in primary care visits was sustained for the 3.5-year follow-up period and that behavioral health outpatient visits began to decrease over time. The estimates produced from our study benefited from the quasi-experimental techniques of IPW and multigroup ITSA. We incorporated area-level socioeconomic characteristics into our propensity score model, which helps estimate the BHH program impact by accounting for variation in outcomes that may be due to area-level factors.
Study Limitations
This study had several limitations. First, all individuals needed at least one encounter in the health system in the pre- and postintervention periods. For the control group, this encounter was for a behavioral health appointment. Individuals may have recorded this encounter early in the postintervention period before leaving the health system’s catchment area. However, results of qualitative sensitivity analyses in which individuals in the control groups required an encounter in 3 separate years postintervention did not influence our primary conclusions (see part 7 of the online supplement). Results were similar in a sensitivity analysis that included later BHH enrollees, although BHH participation for later enrollees was associated with smaller shifts in primary care and behavioral health outpatient utilization compared with shifts among early enrollees (see part 2 of the online supplement). Second, our study mimicked an intent-to-treat analysis. BHH participants were included only if enrolled when implementation began. We did not capture differences for patients enrolled later, and we included in our analyses patients who might have disengaged from the program. Third, subgroup analyses were exploratory because of smaller sample sizes. Fourth, our propensity score model did not control for potential selection bias based on preintervention levels of behavioral health inpatient and outpatient visits. However, not including preintervention levels in the model prevents bias from regression to the mean, which may be strong precisely because of preintervention level differences. Finally, we acknowledge an abrupt decrease in behavioral health inpatient visits for the control population when the intervention began. This decrease may reflect general efforts across the health system to reduce behavioral health hospitalizations in the population. Unexpected changes in real-world data highlight the need for multigroup comparisons when evaluating complex interventions in real-world settings.
Conclusions
This study considered long-term impacts of BHH enrollment on service utilization and cardiometabolic measures and demonstrated increased use of primary care. We found mixed results for long-term health outcomes such as hospitalizations and cardiometabolic values. Future studies should consider barriers to successful BHH implementation among vulnerable subpopulations.
1 : Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015; 72:1172–1181Crossref, Medline, Google Scholar
2 : Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis 2006; 3:A42Medline, Google Scholar
3 : Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001; 58:844–850Crossref, Medline, Google Scholar
4 : Physical health of the long-term mentally ill in the community. Is there unmet need? Br J Psychiatry 1989; 155:777–781Crossref, Medline, Google Scholar
5 : Access to medical care among persons with psychotic and major affective disorders. Psychiatr Serv 2008; 59:847–852Link, Google Scholar
6 : Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006; 86:15–22Crossref, Medline, Google Scholar
7 : Disparities in breast and cervical cancer screening in women with mental illness: a systematic literature review. Am J Prev Med 2013; 44:392–398Crossref, Medline, Google Scholar
8 : Mentally ill Medicare patients less likely than others to receive certain types of surgery. Health Aff 2011; 30:1307–1315Crossref, Google Scholar
9 : Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA 2000; 283:506–511Crossref, Medline, Google Scholar
10 : Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry 2010; 32:519–543Crossref, Medline, Google Scholar
11 : Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry 2007; 68(suppl 4):8–13Crossref, Medline, Google Scholar
12 : The metabolic effects of antipsychotic medications. Can J Psychiatry 2006; 51:480–491Crossref, Medline, Google Scholar
13 : Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry 2006; 51:492–501Crossref, Medline, Google Scholar
14 Parks J, Svendsen D, Singer P, (eds): Morbidity and Mortality in People With Serious Mental Illness. Alexandria, VA,
15 : Behavioral health homes: an opportunity to address healthcare inequities in people with serious mental illness. Asian J Psychiatr 2014; 10:10–16Crossref, Medline, Google Scholar
16 : Improving medical care for persons with serious mental illness: challenges and solutions. J Clin Psychiatry 2007; 68(suppl 4):40–44Crossref, Medline, Google Scholar
17 : A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007; 64:1123–1131Crossref, Medline, Google Scholar
18 : An urgent call to address the deadly consequences of serious mental disorders. JAMA Psychiatry 2015; 72:1166–1167Crossref, Medline, Google Scholar
19 : Evaluation of the SAMHSA Primary and Behavioral Health Care Integration (PBHCI) Grant Program: Final Report (Task 13). Santa Monica, CA,
20 : A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry 2010; 167:151–159Link, Google Scholar
21 : Specialty care medical homes for people with severe, persistent mental disorders. Health Aff 2010; 29:867–873Crossref, Medline, Google Scholar
22 : Creating a ‘reverse’ integrated primary and mental healthcare clinic for those with serious mental illness. Prim Health Care Res Dev 2016; 17:421–427Crossref, Medline, Google Scholar
23 Integrating Primary Care Into Behavioral Health Settings: What Works for Individuals With Serious Mental Illness. New York, Milbank Memorial Fund, 2014. https://www.milbank.org/wp-content/uploads/2016/04/Integrating-Primary-Care-Report.pdfGoogle Scholar
24 : Implementation of integrated health homes and health outcomes for persons with serious mental illness in Los Angeles County. Psychiatr Serv 2016; 67:1062–1067Link, Google Scholar
25 : Integrating primary care into community mental health centers: impact on utilization and costs of health care. Psychiatr Serv 2016; 67:1233–1239Link, Google Scholar
26 : Evolving Models of Behavioral Health Integration: Evidence Update 2010–2015. New York, Milbank Memorial Fund, 2016. https://www.milbank.org/wp-content/uploads/2016/05/Evolving-Models-of-BHI.pdfGoogle Scholar
27 : Descriptive analysis of a novel health care approach: reverse colocation—primary care in a community mental health “home”. Prim Care Companion CNS Disord 2013; 15:PCC.13m01530Medline, Google Scholar
28 : Systematic review of the impact of behavioral health homes on cardiometabolic risk factors for adults with serious mental illness. Psychiatr Serv 2020; 71:57–74Link, Google Scholar
29 : Randomized trial of an integrated behavioral health home: the Health Outcomes Management and Evaluation (HOME) study. Am J Psychiatry 2017; 174:246–255Link, Google Scholar
30 : The effects of the Maryland Medicaid Health Home Waiver on emergency department and inpatient utilization among individuals with serious mental illness. Gen Hosp Psychiatry 2020; 64:99–104Crossref, Medline, Google Scholar
31 : Interventions to increase access to or uptake of physical health screening in people with severe mental illness: a realist review. BMJ Open 2018; 8:e019412Crossref, Medline, Google Scholar
32 Missouri Community Mental Health Center (CMHC) Healthcare Homes Progress Report 2012–2015. Jefferson City, MO, Missouri Department of Mental Health, 2016Google Scholar
33 : Community mental health center integrated care outcomes. Psychiatr Q 2018; 89:969–982Crossref, Medline, Google Scholar
34 : Mind the gap: developing an integrated behavioral health home to address health disparities in serious mental illness. Psychiatr Serv 2017; 68:1217–1224Link, Google Scholar
35 : Multiple Imputation for Survey Nonresponse. New York, Wiley, 1987Crossref, Google Scholar
36 : Applying a propensity score-based weighting model to interrupted time series data: improving causal inference in programme evaluation. J Eval Clin Pract 2011; 17:1231–1238Crossref, Medline, Google Scholar
37 : Matching and regression to the mean in difference-in-differences analysis. Health Serv Res 2018; 53:4138–4156Crossref, Medline, Google Scholar
38 : Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013; 6:604–611Crossref, Medline, Google Scholar
39 Stata Statistical Software, Release 15. College Station, TX, StataCorp, 2017Google Scholar
40 : Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J 2015; 15:480–500Crossref, Google Scholar
41 : Physical health outcomes and implementation of behavioural health homes: a comprehensive review. Int Rev Psychiatry 2018; 30:224–241Crossref, Medline, Google Scholar
42 : Medicare payment for behavioral health integration. N Engl J Med 2017; 376:405–407Crossref, Medline, Google Scholar
43 : Moving Toward Value-Based Payment for Medicaid Behavioral Health Services. Hamilton, NJ, Center for Health Care Strategies, 2017Google Scholar
44 : Cost savings associated with an alternative payment model for integrating behavioral health in primary care. Transl Behav Med 2019; 9:274–281Crossref, Medline, Google Scholar
45 : Behavioral Health Integration into primary care: a microsimulation of financial implications for practices. J Gen Intern Med 2017; 32:1330–1341Crossref, Medline, Google Scholar
46 : The cost effectiveness of embedding a behavioral health clinician into an existing primary care practice to facilitate the integration of care: a prospective, case-control program evaluation. J Clin Psychol Med Settings 2019; 26:59–67Crossref, Medline, Google Scholar



