Cost-Effectiveness of Long-Acting Injectable Paliperidone Palmitate Versus Haloperidol Decanoate in Maintenance Treatment of Schizophrenia
Abstract
Objective:
This study assessed the relative cost-effectiveness of haloperidol decanoate (HD), a first-generation long-acting injectable (LAI) antipsychotic, and paliperidone palmitate (PP), a second-generation LAI antipsychotic.
Methods:
A double-blind, randomized 18-month clinical trial conducted at 22 clinical research sites in the United States compared the cost-effectiveness of HD and PP among 311 adults with schizophrenia or schizoaffective disorder who had been clinically assessed as likely to benefit from an LAI antipsychotic. Patients were randomly assigned to monthly intramuscular injections of HD (25–200 mg) or PP (39–234 mg) for up to 24 months. Quality-adjusted life years (QALYs) were measured by a schizophrenia-specific algorithm based on the Positive and Negative Syndrome Scale and side-effect assessments; total health care costs were assessed from the perspective of the health system.
Results:
Mixed-model analysis showed that PP was associated with .0297 greater QALYs over 18 months (p=.03) and with $2,100 more in average costs per quarter for inpatient and outpatient services and medication compared with HD (p<.001). Bootstrap analysis with 5,000 replications showed an incremental cost-effectiveness ratio for PP of $508,241 per QALY (95% confidence interval=$122,390–$1,582,711). Net health benefits analysis showed a .98 probability of greater cost-effectiveness for HD compared with PP at an estimated value of $150,000 per QALY and a .50 probability of greater cost-effectiveness at $500,000 per QALY.
Conclusions:
HD was more cost-effective than PP, suggesting that PP’s slightly greater benefits did not justify its markedly higher costs, which are likely to fall once the medication’s patent expires.
Nonadherence to prescribed medication is a major cause of relapse, rehospitalization, and increased health care costs in the treatment of schizophrenia (1). Long-acting injectable (LAI) antipsychotic medications, which can be administered every two to four weeks, are used to reduce nonadherence and relapse. The use of LAI versions of first-generation antipsychotics has been limited, in part because of concerns about the risk of extrapyramidal side effects. Risperidone microspheres, an LAI version of the second-generation antipsychotic risperidone, was introduced in 2003, but it must be refrigerated before use, reconstituted with a diluent, and administered every two weeks. In 2009, paliperidone palmitate (PP), an LAI version of risperidone’s active metabolite, became available. It can be administered monthly and does not require refrigeration or reconstitution. Because of these logistical advantages, PP was considered to be an important advance in LAI antipsychotics (2), although its high cost has raised uncertainty about whether its costs are justified by greater benefits.
Recent trials have raised some doubts about the clinical advantages of oral second-generation antipsychotics compared with first-generation oral antipsychotics (3–6). Some newer antipsychotics appear to cause significant metabolic problems (7), and randomized trials have failed to find advantages of LAI versions of second-generation antipsychotics compared with oral formulations of the same drugs (8,9). However, one recent study found robust benefits for LAI versus oral risperidone in first-episode psychosis (10).
In view of these uncertainties, a randomized controlled trial was designed to compare PP and the first-generation LAI antipsychotic haloperidol decanoate (HD). The study found that PP provided no advantage in preventing relapse but was associated with greater weight gain and reduced risk of akathisia (11). This study evaluated the cost-effectiveness of PP and HD by measuring the relative effectiveness of PP and HD with respect to health status, including both symptoms and side effects, of people with schizophrenia and analyzing whether any health advantage provided by PP merits its greater cost.
Methods
Study Setting and Design
The study, Comparison of Long-Acting Injectable Medications for Schizophrenia, was a multisite, parallel-group, double-blinded randomized controlled trial (RCT) conducted at 22 U.S. clinical sites from 2011 to 2013 (11). Each site obtained institutional review board approval to conduct the study. A Data and Safety Monitoring Board convened by the National Institute of Mental Health monitored the study.
Patients
Patients were adults ages 18–65 with a diagnosis of schizophrenia or schizoaffective disorder confirmed by the Structured Clinical Interview for DSM-IV (SCID). Patients were eligible if they had been judged by a referring psychiatrist as likely to benefit from treatment with PP or HD because they were at risk of experiencing efficacy failure due to medication noncompliance or significant substance abuse. Entry and exclusion criteria have been presented previously (11).
Interventions
A total of 353 patients enrolled for screening; 311 were eligible and were randomly assigned to study treatment (11). Study treatments were PP supplied in dosages of 39 mg, 78 mg, 117 mg, 156 mg, and 234 mg and HD supplied in vials of 50 mg/ml or 100 mg/ml for injection. Each participant received a blinded trial of the oral version of the assigned medication prior to receiving an injection. The first injection was given four to seven days after the baseline visit. Subsequent visits were at weeks 1, 2, 4, 6, 8, 10, and 12, followed by monthly (every four weeks) visits for up to 24 months. Altogether, 62 of 145 (43%) PP patients and 71 of 145 (49%) HD patients completed the 18-month follow-up assessment, with no significant difference between the groups’ completion rates (χ2=.77, df=6, p=.94).
Study physicians and all other personnel were blinded to treatment condition. A clinician not otherwise involved in the trial administered the injection (11).
Outcome Measures
The primary outcomes were quality-adjusted life years (QALYs) and total health care costs from the perspective of the health care system (12).
Effectiveness.
Cost-effectiveness analysis depends on having a single measure of health-related quality of life that addresses health gains as well as losses due to side effects. It is recommended that health states be expressed as QALYs, a year of life rated on a cardinal scale from 0 (worst possible health) to 1 (perfect health), as evaluated by members of the general public (12).
A series of studies has demonstrated a method for evaluating QALYs for schizophrenia (13–15) on the basis of the Positive and Negative Syndrome Scale (PANSS) (16) and side-effect data. The derivation of QALYs from PANSS data began with a cluster analysis of a sample of almost 400 patients, which identified eight disease-specific health states. With input from expert study clinicians, a script and video materials were developed to convey impairments associated with each schizophrenia state and with five commonly co-occurring adverse side effects (orthostatic hypotension, weight gain, tardive dyskinesia, pseudo-parkinsonism, and akathisia) (13). These video presentations were viewed by 620 members of the general public, who rated each state by using the standard gamble, the recommended method for QALY determination (12). Responses were weighted to represent the sociodemographic characteristics of the adult U.S, population.
Service use and costs.
The economic perspective addressed total health care costs (use of mental health and general medical services plus medications at prices faced by the health care system, the perspective used in this study). Trained research staff conducted detailed quarterly interviews to assess service use, documenting inpatient and outpatient psychiatric and general medical service use. Costs were then estimated by multiplying the number of units of each type of service received by the estimated unit cost of that service and then summing the products across different services.
Monthly service use was documented every three months through a self-report questionnaire that recorded three kinds of hospital days (medical, surgical, and psychiatric and substance abuse) across six different facility types, for example, state mental hospitals, private psychiatric hospitals, and nonfederal general hospitals. Nights spent in nursing homes and halfway houses were also recorded. Use of 16 types of outpatient mental health care, including psychiatric and psychosocial rehabilitation services, was documented along with use of eight different types of medical or surgical outpatient visits and emergency room services.
Unit costs of these services were estimated from published reports (17–22) and administrative data sets (Medicaid, MarketScan private claims database, and Veterans Health Administration data) (22).
Costs of antipsychotic medications, other psychotropic medications, and nonpsychotropic medication were based on discounted prices from the Federal Supply Schedule, the lowest prices available for nongeneric medications, ensuring that the cost-effectiveness results represent an analysis of the most conservative prices. The unit of analysis for cost evaluation was the total average health cost per quarter (average monthly costs × 3), including costs of all health service use, study medications at the prescribed doses, and other prescribed drugs.
Statistical Methods
The analytic sample consisted of all patients who received at least one injection and at least one postbaseline assessment.
Effectiveness, service use, and cost analyses compared treatment groups on average quarterly measures of effectiveness and service use (QALYs, hospital or residential days, outpatient visits, and medications) and related costs across 18 months by using a mixed model including terms representing treatment group and time (treated as a classification variable for three, six, nine, 12, 15, and 18 months). A random-subject effect and a first-order autoregressive covariance structure were used to adjust standard errors for the correlation of observations from the same individual.
In addition to the comparison between the treatments on the bases of effectiveness (improvement in QALYs from baseline) and costs, an incremental cost-effectiveness ratio (ICER) was calculated as the difference in benefits divided by the difference in quarterly costs. The uncertainty of estimated differences in cost and effectiveness was estimated by nonparametric bootstrapping of ICERs for PP compared with HD by using 5,000 regression replications with replacement (23).
The principal cost-benefit analysis was conducted by using the method of net health benefits (24). In this approach, a range of estimates for the dollar value of a QALY is multiplied by the QALY estimate for each patient at each time point to estimate the monetized value of the patient’s health status at each observation. Following conventions used in policy making (25,26), including recent refinements of the conventions by academic researchers, we used estimates of $0 to $600,000 per QALY per year in this sensitivity analysis. This yielded a monetized estimate of health status for each patient at each time point.
Monthly health care costs were then subtracted from these estimated health benefits to generate an estimate of “net health benefit” per patient per month for each of the estimated monetary values of a QALY. Mixed-model regression analyses of the type described above were used to compare mean differences between the groups by using monthly estimates of net health benefits from all time points and adjusting for time, site, and other factors.
Over the past decade, it has been increasingly recognized that policy makers typically must make decisions even when findings do not meet the usual 5% standard of uncertainly. It is important to know the probability that one treatment will be more cost-effective than another, even when the uncertainty is greater than the conventional 5% (23). Using the method of Hoch and others (27), we calculated the probability that HD had greater net health benefits compared with PP at each of the estimated monetary values of a QALY. This calculation was based on a one-tailed test in which the p value was associated with the coefficient for the treatment variable, representing the significance of differences between the treatments (calculated as HD–PP), and was computed as 1–p/2 (27). These data allow plotting of a cost-effectiveness acceptability curve, which illustrates graphically the probability that HD was more cost-effective than PP at each estimated monetary value of a QALY.
Analyses were performed by using SAS, version 9.3.
Results
Of the 311 patients who were screened and randomly assigned to each group, 290 patients (PP, N=145; HD, N=145) received at least one injection and at least one postbaseline assessment and were included in the primary analysis of the original study (11). Baseline demographic and clinical characteristics of the 290 patients in the primary analysis are presented in the earlier article (11).
Dose
In the initial month of LAI treatment, which included doses on day 1 and day 8, the mean dose was 325 mg for PP and 94 mg for HD. Subsequently, the mean monthly dose of PP ranged from 129 to 169 mg and the mean monthly dose of HD ranged from 67 to 83 mg.
Summary of Previously Published Results
In the primary analysis of the original study, there was no statistically significant difference in the time to efficacy failure for patients taking PP (49 days) or HD (47 days) (site-stratified log rank p=.90; adjusted hazard ratio=.98, 95% confidence interval [CI]=.65–1.47, adjusted for site and baseline PANSS score). On average, patients taking PP gained weight progressively over time and those on HD lost weight (p<.001).
Patients taking HD experienced more akathisia compared with patients taking PP, as indicated by greater increases in average (SD) global scores on the Barnes Akathisia Scale (.73 [.59–.87] and .45 [.31–.59], respectively, p=.006). There were no statistically significant differences between groups at each time point in changes in ratings of parkinsonism (measured by the Simpson-Angus Extrapyramidal Scale) and in decreases in PANSS total scores compared with baseline.
QALYs
Mixed-model analysis of QALYs as measured by the algorithm described above showed that scores for PP were slightly higher compared with scores for HD, with an average difference of .0297 QALYs over 18 months (p<.03) (Figure 1).
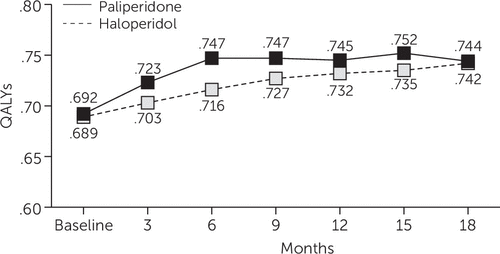
FIGURE 1. Quality-adjusted life years (QALYs) among individuals with schizophrenia who were treated with paliperidone palmitate (PP) or haloperidol decanoate (HD) over an 18-month study period, by quartera
aQALYs are rated on a cardinal scale from 0, indicating worst possible health, to 1, indicating perfect health. QALYs for individuals using PP were .0297 higher, on average, than QALYs for individuals using HD (p<.03).
Costs
Antipsychotic drug costs were $2,213 greater per quarter for the PP group compared with the HD group (p<.001) (Table 1). There were no significant differences between the two groups in other medication costs. Nor were there any significant differences between the treatment groups in total inpatient and outpatient mental health and medical-surgical services use or related service costs (excluding medications) (Table 1). The average total costs per quarter for services and medications during the 18-month follow-up period were $2,100 greater for the PP group compared with the HD group (p=.003) (Table 1). Figure 2 depicts total costs per quarter for both groups.
| PP | HD | Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SE | Mean | SE | Mean | SE | Z | p |
| Service use | ||||||||
| Inpatient or residential (days) | 4.98 | .63 | 4.99 | .63 | –.01 | .98 | .54 | .59 |
| Mental health inpatient | .19 | .04 | .26 | .06 | –.07 | .15 | .64 | .52 |
| Residential or nursing home | 4.53 | .64 | 4.69 | .6 | .–16 | .93 | .94 | .35 |
| Medical-surgical inpatient | .09 | .03 | .07 | .03 | .02 | .09 | 1.5 | .13 |
| Outpatient (visits) | 4.00 | .39 | 4.40 | .46 | –.40 | .99 | .41 | .68 |
| Emergency department | .10 | .03 | .06 | .008 | .04 | .02 | 1.4 | .16 |
| Mental health outpatient | 3.48 | .37 | 3.89 | .43 | –.41 | .93 | .72 | .47 |
| Medical-surgical outpatient | .42 | .04 | .45 | .06 | –.03 | .11 | .21 | .84 |
| Costs for services, excluding medications ($) | ||||||||
| Total | 3,654 | 488 | 3,754 | 428 | –100 | 1,081 | .38 | .70 |
| Inpatient or residential treatment | 2,367 | 452 | 2,316 | 349 | 51 | 1,023 | .47 | .64 |
| All mental health and medical-surgical inpatient services | 1,441 | 365 | 1,470 | 299 | –29 | 956 | .31 | .76 |
| Residential and nursing home services | 877 | 127 | 860 | 114 | 17 | 166 | 1.09 | .27 |
| Outpatient services | 1,315 | 138 | 1,413 | 150 | –98 | 297 | .15 | .88 |
| Emergency department | 40 | 9.6 | 25 | 3.1 | 15 | 9 | 1.44 | .15 |
| Medical-surgical outpatient services | 120 | 12 | 130 | 16 | –10 | 29 | .21 | .84 |
| Mental health outpatient services | 1,157 | 134 | 1,264 | 144 | –107 | 287 | .16 | .87 |
| Total medication costs ($) | 3,770 | 135 | 1,653 | 112 | 2,117 | 173 | 17 | <.001 |
| Study medication | 2,275 | 116 | 62 | 3.6 | 2,213 | 105 | 26.7 | <.001 |
| Other psychotropic medications | 591 | 55 | 572 | 58 | 19 | 70 | 1.26 | .21 |
| Nonpsychotropic drugs | 934 | 49 | 1,027 | 79 | –93 | 107 | .47 | .64 |
| Total costs (services plus medications) ($) | 7,545 | 521 | 5,445 | 484 | 2,100 | 1,098 | 2.94 | .003 |
TABLE 1. Use and costs of services per quarter over 18 months among users of paliperidone palmitate (PP) and haloperidol decanoate (HD)a
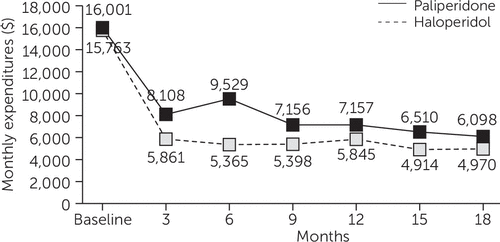
FIGURE 2. Total outpatient, inpatient, and drug costs for individuals with schizophrenia who were treated with paliperidone palmitate (PP) or haloperidol decanoate (HD) over an 18-month study period, by quartera
a Total costs were significantly higher for individuals treated with PP than for individuals treated with HD (p<.003).
Cost-Effectiveness and Net Health Benefits
Dividing incremental costs by incremental benefits in the bootstrap analysis generated an ICER of $508,241 per QALY for PP compared with HD (Figure 3), with 98% of observations falling in the upper-right quadrant of the cost-effectiveness plane, indicating greater benefits for PP as well as greater costs.
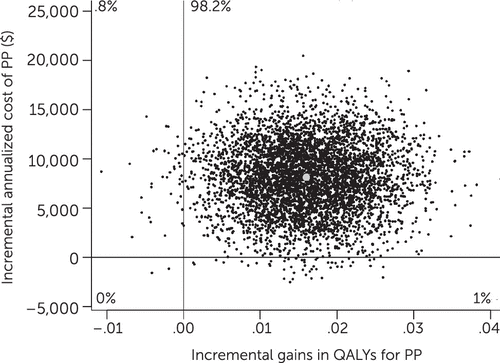
FIGURE 3. Bootstrap analysis of the incremental cost-effectiveness ratio (ICER) for treatment with paliperidone palmitate (PP) versus haloperidol decanoate (HD)a
aPP was associated with a .016 improvement in quality-adjusted life years (QALYs) and $8,133 in higher quarterly costs compared with HD, for an ICER of $508,241 per QALY (95% confidence interval=$122,390–$1,582,711); 98.2% of observations fell in the upper-right quadrant of the cost-effectiveness plane, indicating greater benefits for PP as well as greater costs. QALYs are rated on a cardinal scale from 0, indicating worst possible health, to 1, indicating perfect health.
Analysis of net health benefits expressed in dollars at each estimated value of a QALY showed that HD had a .95 probability of being more cost-effective compared with PP at QALY values less than $150,000 and a .81 probability of being more cost-effective compared with PP at a QALY value of $300,000 (Figure 4). The probability that HD was more cost-effective than PP declined steadily at QALY values greater than $300,000 (Figure 4), falling to .50 at a QALY value of $500,000 and to .44 at a QALY value of $600,000.
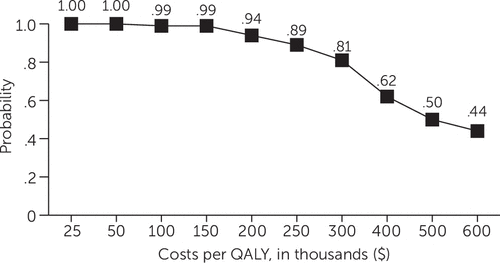
FIGURE 4. Probability of greater cost-effectiveness of treatment with haloperidol decanoate versus paliperidone palmitate, by cost per quality-adjusted life year (QALY)
Discussion
This study used a disease-specific method to calculate QALYs associated with various health states among people diagnosed as having schizophrenia. It found that treatment with PP resulted in a statistically significant but small advantage in health status. However, because PP remains on patent and has relatively high acquisition costs, it is not a cost-effective treatment choice under a wide range of estimates of the monetary value of a QALY. That was true even though we used the lowest available estimate for the cost of the drug. For example, on the basis of the Federal Supply Schedule, 117 mg of PP was priced at $758 compared with a published average wholesale price of $889 and online retail prices ranging from $1,023 to $1,106 (http://www.goodrx.com/paliperidone-palmitate#/?filter location=&coords=&label=Invega+Sustenna&form=syringe&strength=0.75ml+of+117mg&quantity=1.0&qty-custom) as of July 16, 2015. Thus our findings would be sustained under a wide range of 2015 prices for PP, although prices are likely to drop when generic versions of the drug appear after patent protection runs out in 2017.
Many studies of newer second-generation antipsychotics end up with ambiguous findings showing that some side effects are less severe with new treatments, but others are more severe. The QALY measure used in this study (13) provides a rational approach to combining data on symptoms and major side effects in a single measure. In addition, selective inclusion of only individuals judged by their clinicians to be likely to benefit from LAI treatment, who most closely resemble patients prescribed LAI treatments in real-world practice, enhanced the study’s external validity.
There has been controversy about the monetary value that should be assigned to a QALY in cost-benefit analysis. On the one hand, some governments have long used $50,000 as the appropriate value of a QALY for policy making (25). However, this estimate was first established in 1982 (26), and by 2011, when this study was initiated, it would have reached $117,000 just by adjusting for inflation alone. A more recent valuation of a QALY suggested a range from $183,000 to $264,000. This valuation was based on an empirical estimate of the cost-effectiveness of treatments available in 2003 compared with care available in 1950 and of the implicit cost-effectiveness of unsubsidized insurance compared with self-pay care.
Although HD was clearly more likely to be cost-effective compared with PP at a QALY valuation of $50,000 (.998 probability) and at an inflation-adjusted valuation of $117,000 (.985 probability), it also had a .94 probability of being more cost-effective at a QALY value of $200,000 (the lower bound of the estimate by Braithwaite and others [26]) and a .81 probability of being more cost-effective at a QALY value of $300,000 (the upper-bound estimate by Braithwaite and others [26]). The probability that HD was more cost-effective than PP declined only at values of $500,000 and $600,000 per QALY, far higher than the values that are currently accepted. Thus HD was substantially more likely than PP to be cost-effective at a broad range of estimates of the value of a QALY as well as of the cost of PP.
The results of this study are consistent with the only other RCT-based cost-effectiveness study of an LAI second-generation antipsychotic. That study found that LAI risperidone (8) was not associated with significantly greater benefits on multiple measures compared with a doctor’s choice of oral medication. However, the LAI treatment was associated with more neurological side effects and significantly increased drug costs. Total health care costs were 7% greater among the users of LAIs compared with users of oral medication, but the difference was not statistically significant (28).
Several RCTs have found no benefit of LAI second-generation antipsychotics compared with oral antipsychotics (29–32). Two studies found positive benefits for LAI risperidone (9,33), and a recent publication found robust benefits for LAI risperidone compared with oral risperidone for patients experiencing a first episode of psychosis (10). Although there is mixed evidence of the superiority of second-generation antipsychotic LAIs compared with oral medications, the use of LAIs is supported by systematic reviews (34,35) and expert panels (36). This study is the only RCT to compare the cost-effectiveness of first- and second-generation LAIs.
Given that several newer LAI antipsychotics are now on the market in the United States with high acquisition costs associated with being on patent, total national expenditures on these drugs may rise rapidly. The results of this study should encourage consideration of older, less expensive drugs, such as HD. Used at moderate dosages in this study, HD’s overall effectiveness and tolerability were only slightly worse, as reported here, than those of PP, and it had clear advantages in cost-effectiveness. When generic versions of the newer LAIs become available, the cost-effectiveness calculations will undoubtedly change. In the meantime, trial evidence appears to indicate that HD is a cost-effective choice. A rational policy for treatment of chronic schizophrenia might limit use of the more expensive LAIs to patients who do not benefit from or cannot tolerate HD.
Several methodological limitations require comment. The QALY algorithm used in this study has not been further validated since its initial validation. It must be acknowledged that people with serious mental illness may have difficulty responding to standard gamble choices, even when they are presented with simplified graphic displays. In addition, although the QALY responses were weighted by using sociodemographic characteristics of the U.S. population, QALY values may vary by unmeasured respondent characteristics. Imperfect as this measure may be, the results were consistent with those observed on individual measures in the original article from this study (11) and provide a rational empirical basis for assigning monetary values to health states.
Second, utilization data were based on self-report estimations by patients of service use and published estimations of unit costs rather than on verified service use or actual unit costs, and there were substantial amounts of missing data from the study. If respondents underestimated their service use, which might be expected as a result of memory lapses, actual group differences could have been underestimated as well. Missing data were addressed in the mixed models by using all available data, and study dropout rates were similar to those in the Clinical Antipsychotic Trials of Intervention Effectiveness study by the National Institute of Mental Health (5,37) and others (37), but recall biases cannot be ruled out. In spite of these limitations, the findings of this study appear to be robust in a number of sensitivity analyses representing alternative assumptions and methods of analysis.
Conclusions
HD was more cost-effective than PP, suggesting that PP’s slightly greater benefits do not justify the markedly higher costs associated with its status as an on-patent medicine.
1 : An economic review of compliance with medication therapy in the treatment of schizophrenia. Psychiatric Services 54:508–516, 2003Link, Google Scholar
2 : Paliperidone palmitate: review of the efficacy, safety and cost of a new second-generation depot antipsychotic medication. International Journal of Clinical Practice 64:216–239, 2010Crossref, Medline, Google Scholar
3 : Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Archives of General Psychiatry 63:1079–1087, 2006Crossref, Medline, Google Scholar
4 : Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. JAMA 290:2693–2702, 2003Crossref, Medline, Google Scholar
5 : Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209–1223, 2005Crossref, Medline, Google Scholar
6 : Noninferiority of perphenazine vs three second-generation antipsychotics in chronic schizophrenia. Journal of Nervous and Mental Disease 202:18–24, 2014Crossref, Medline, Google Scholar
7 : Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 19(suppl 1):1–93, 2005Crossref, Medline, Google Scholar
8 : Long-acting risperidone and oral antipsychotics in unstable schizophrenia. New England Journal of Medicine 364:842–851, 2011Crossref, Medline, Google Scholar
9 : Comparison of SGA oral medications and a long-acting injectable SGA: the PROACTIVE study. Schizophrenia Bulletin 41:449–459, 2015Crossref, Medline, Google Scholar
10 : Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia: a randomized clinical trial. JAMA Psychiatry 72:822–829, 2015Crossref, Medline, Google Scholar
11 : Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 311:1978–1987, 2014Crossref, Medline, Google Scholar
12 : Cost Effectiveness in Health and Medicine. New York, Oxford University Press, 1996Crossref, Google Scholar
13 : Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizophrenia Research 71:155–165, 2004Crossref, Medline, Google Scholar
14 : Toward improved methods for measurement of utility: automated repair of errors in elicitations. Medical Decision Making 23:67–75, 2004Crossref, Google Scholar
15 : The heterogeneity of schizophrenia in disease states. Schizophrenia Research 71:83–95, 2004Crossref, Medline, Google Scholar
16 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 13:261–276, 1987Crossref, Medline, Google Scholar
17 SMNHA Mental Health—Controlled Expenditures Per Inpatient Day, All Civil (Voluntary and Involuntary) Patients in State Psychiatric Hospitals Receiving Mental Health Services by Age and State, FY 2002. Alexandria, Va, National Association of State Mental Health Program Directors, 2002Google Scholar
18 : Recent trends in state nursing home payment policies. Health Affairs (suppl Web Exclusives):W4-363–373, 2004Crossref, Google Scholar
19 The ADSS Cost Study: Costs of Substance Abuse Treatment in the Specialty Sector. DHHS pub no SMA 03-3762, Analytic Series A-20. Rockville, Md, Substance Abuse and Mental Health Services Administration, 2004Google Scholar
20 Kasprow WJ, Robert R, DiLella D, et al: Health Care for Homeless Veterans Programs: The Seventeenth Annual Report. West Haven, Conn, US Department of Veterans Affairs, North East Program Evaluation Center, 2004Google Scholar
21 : Review of methods to determine VA health care costs. Medical Care 37:AS9–AS17, 1999Crossref, Medline, Google Scholar
22 : National Mental Health Program Performance Monitoring System: Fiscal Year 2002 Report. West Haven, Conn, Northeast Program Evaluation Center, 2003Google Scholar
23 : Confidence intervals or surfaces? Uncertainty on the cost-effectiveness plane. Health Economics 7:723–740, 1998Crossref, Medline, Google Scholar
24 : Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Medical Decision Making 18:S68–S80, 1998Crossref, Medline, Google Scholar
25 : Using Cost-Effectiveness Analysis to Improve Health Care. New York, Oxford University Press, 2005Google Scholar
26 : What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Medical Care 46:349–356, 2008Crossref, Medline, Google Scholar
27 : Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Economics 11:415–430, 2002Crossref, Medline, Google Scholar
28 : Cost and cost-effectiveness in a randomized trial of long-acting risperidone for schizophrenia. Journal of Clinical Psychiatry 73:696–702, 2012Crossref, Medline, Google Scholar
29 : Equivalent switching dose from oral risperidone to risperidone long-acting injection: a 48-week randomized, prospective, single-blind pharmacokinetic study. Journal of Clinical Psychiatry 68:1218–1225, 2007Crossref, Medline, Google Scholar
30 : Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. European Neuropsychopharmacology 15:111–117, 2015Crossref, Google Scholar
31 : Long-acting injectable risperidone v olanzapine tablets for schizophrenia or schizoaffective disorder: randomised, controlled, open-label study. British Journal of Psychiatry 191:131–139, 2007Crossref, Medline, Google Scholar
32 : A prospective study comparing the long-term effectiveness of injectable risperidone long-acting therapy and oral aripiprazole in patients with schizophrenia. Psychiatry 7:23–31, 2010Medline, Google Scholar
33 : Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long-acting injectable vs quetiapine: results of a long-term, open-label, randomized clinical trial. Neuropsychopharmacology 35:2367–2377, 2010Crossref, Medline, Google Scholar
34 : Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. Journal of Clinical Psychiatry 74:957–965, 2013Crossref, Medline, Google Scholar
35 : Oral versus depot antipsychotic drugs for schizophrenia—a critical systematic review and meta-analysis of randomised long-term trials. Schizophrenia Research 127:83–92, 2011Crossref, Medline, Google Scholar
36 : The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. Journal of Clinical Psychiatry 68:1751–1762, 2007Crossref, Medline, Google Scholar
37 : Second-generation antipsychotics: reviewing the cost-effectiveness component of the CATIE trial. Expert Review of Pharmacoeconomics and Outcomes Research 7:103–111, 2007Crossref, Medline, Google Scholar



