Risk of Dementia Among Elderly Nursing Home Patients Using Paroxetine and Other Selective Serotonin Reuptake Inhibitors
Abstract
Objective:
Selective serotonin reuptake inhibitors (SSRIs) are the first line of treatment for depression. Among the SSRIs, paroxetine has strong anticholinergic properties and may lead to increased risk of adverse cognitive outcomes among elderly patients. This study evaluated the comparative risk of dementia associated with use of paroxetine and other SSRIs among elderly nursing home patients.
Methods:
A retrospective cohort study using propensity score matching was conducted with 2007–2010 Minimum Data Set–linked Medicare data. The study population included elderly nursing home patients with depression who were new users of SSRIs. Patients were followed for a maximum of two years after index SSRI use. The risk of dementia was modeled by using a robust Cox proportional hazards model to account for clustering within matched users of paroxetine and other SSRIs.
Results:
The unmatched cohort included 19,952 new users of SSRIs; 1,898 used paroxetine, and 18,054 used other SSRIs. In the propensity-matched cohort of 3,796 patients, the unadjusted incidence of dementia was 7.5% for users of paroxetine and 8.6% for users of other SSRIs. There was no difference in the risk of dementia for users of paroxetine or other SSRIs. These study findings remained robust in multiple sensitivity analyses involving various measures of dementia.
Conclusions:
Compared with use of other SSRIs, use of paroxetine was not associated with higher risk of dementia among elderly nursing home patients with depression. Future studies are needed to evaluate the impact of paroxetine on other cognition measures.
Considered one of the most critical public health concerns in the United States, dementia is a general term for a group of disorders that causes progressive deterioration in cognitive functioning. Alzheimer’s disease accounts for 50% to 60% of dementia cases (1); other types of dementia include those due to Lewy body disease, vascular disease, mixed etiologies, and frontotemporal lobar degeneration (2,3). About 5.3 million people in the United States have Alzheimer’s disease, the nation’s seventh leading cause of death (4,5). Available drugs for treatment of dementia, such as cholinesterase inhibitors and memantine, offer small benefit and do not alter the disease’s progression (6). Consequently, prevention of dementia through identification and modification of risk factors is the key to reduce the disease burden until new disease-reversing agents are proved efficacious. It is estimated that the prevalence of dementia could be reduced by 50% if risk reduction strategies were successful in delaying onset of the disease by five years (7).
Depression is a major risk factor for cognitive decline and dementia. Ownby and colleagues (8) found that having depression was associated with significantly higher odds of Alzheimer’s disease in case-control (odds ratio [OR]=2.0) and cohort (OR=1.9) studies. A meta-analysis by Jorm and Jolley (9) found that depression was consistently associated with an increased risk of dementia in both case-control studies (95% confidence interval [CI] for relative risk [RR]=1.16–3.50) and prospective studies (CI for RR=1.08–3.20). In addition, a meta-analysis by Christensen and colleagues (10) revealed that depressive patients had low performance on almost all cognitive tests. A recent report by the Agency for Healthcare Research and Quality (11) found increased risk of cognitive decline among depressed patients; the report was based on 13 studies with a follow-up of 1.5 to 5.6 years.
Previous literature suggests a strong association between anticholinergic use and risk of cognitive decline and dementia (12–14). Campbell and colleagues (14) reviewed ten studies and found a consistent association between anticholinergics and cognitive impairment, which was defined as worsening of dementia, a new diagnosis of dementia, or global decline in cognition. Carrière and others (13) found that the use of anticholinergic medications in a prospective cohort of elderly patients was associated with cognitive decline during a follow-up period of four years. A high risk of incident dementia was also observed among continuous users of anticholinergics (hazard ratio [HR]=1.65, CI=1.00–2.73) but not among those who discontinued the medications. Another longitudinal study, the Indianapolis-Ibadan dementia project, revealed that number of anticholinergics was associated with an increased risk of cognitive impairment (OR=1.46, CI=1.07–1.99) (12).
Selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors are considered first-line agents for treatment of depression (15,16). A recent comparative-effectiveness review found that second-generation antidepressants do not substantially differ from each other in terms of efficacy (17). Adverse events and onset of action should be primary considerations in selecting second-generation antidepressants (17,18). SSRIs vary in their pharmacodynamic and pharmacokinetic profiles, despite having the same principal mechanism of action (19). Paroxetine was found to have higher affinity for muscarinic acetylcholine receptors compared with other SSRIs (20,21). Owen and others (21) found that paroxetine’s muscarinic binding was similar to that of desipramine but much lower compared with amitriptyline and substantially higher compared with sertraline. Thus paroxetine’s anticholinergic side effects are expected to be higher compared with other SSRIs. Goodnick and Goldstein (19) suggested that all SSRIs except paroxetine are devoid of anticholinergic properties. Studies suggest that paroxetine is a muscarinic antagonist and has a less favorable tolerability profile compared with escitalopram (20). The American Geriatrics Society (22) considers use of paroxetine for patients with impaired cognition to be potentially inappropriate because of the drug’s strong anticholinergic properties.
Although paroxetine is highly anticholinergic, it is commonly used for the treatment of depression among elderly patients (23–25). However, very little is known about its cognitive effects among elderly patients with depression. None of the previous studies have examined paroxetine use and the risk of dementia among elderly patients with depression, especially in nursing homes. There is an evidence gap regarding the safety and effectiveness of medications used by nursing home residents because of the practical considerations related to treatment ethics, data access, and research costs (26). Thus the purpose of this study was to evaluate the risk of dementia associated with use of paroxetine versus other SSRIs among elderly nursing home patients with depression.
Methods
Data Source
The study used 2007–2010 Minimum Data Set (MDS)–linked Medicare data files obtained from the Chronic Conditions Data Warehouse (CCW) to examine the risk of dementia associated with use of paroxetine versus other SSRIs by elderly nursing home patients with depression. The CCW files are available as Research Identifiable Files (RIFs). The Medicare Provider Analysis and Review file (Part A), the prescription claims file (Part D), the MDS, and the Master Beneficiary Summary File (MBSF) were used in this study. The chronic condition (CC) segment of the MBSF was included; it contains information from inpatient and outpatient claims data on 27 common chronic conditions (27,28). The base cohort included Medicare beneficiaries with depression, identified on the basis of the MBSF CC segment and with a MDS assessment during the study period. This study was approved by the University of Houston Committee for the Protection of Human Subjects under the exempt category.
Study Design
The study design involved a retrospective cohort matched on propensity scores. Index use of SSRI antidepressant was defined as having first prescription of SSRI antidepressant without any antidepressant use in the one-year baseline period, as indicated by Part D claims. [A schematic presentation of cohort construction is available as an online supplement to this article.]
Selected patients were individuals who resided in a nursing home anytime during the study period; were age 65 or older; had been diagnosed as having depression during a one-year baseline period; had initiated SSRIs between January 1, 2008, and December 31, 2008; were continuously eligible for Medicare Parts A, B, and D during the one-year baseline period; and were not diagnosed as having dementia during the baseline period. [Details about the development of the study cohort are available in the online supplement.] Patients enrolled in a health maintenance organization during the study period were excluded because predefined indicators of chronic conditions were obtained only from the claims files of fee-for-service beneficiaries and not from managed care organizations (29,30).
Exposure and Outcome Definitions
Index SSRI exposure was measured by using prescription Medicare Part D claims data. SSRIs were classified into paroxetine and other SSRIs. Other SSRIs included sertraline, citalopram, fluoxetine, fluvoxamine, and escitalopram. National Drug Codes in the Part D file were used to identify SSRI exposure (31). The primary outcome measured was time to diagnosis of dementia after initiation of either paroxetine or other SSRIs. Diagnosis of dementia was based on the CC segment of the MBSF. Study participants were censored if they reached the end of the two-year follow-up period, had switched to a different antidepressant class, had switched from paroxetine to other SSRIs or vice versa, had a gap of more than 15 days in the use of the index antidepressant (32–34), or had died, whichever occurred earlier. Due to the pharmacokinetic and pharmacodynamic properties of antidepressants, a diagnosis of dementia within 30 days of taking an antidepressant cannot necessarily be attributed to antidepressant treatment. Therefore, the diagnosis of dementia was not evaluated for at least 30 days after the start of antidepressant treatment (15).
Cohort Matching
The strength of observational studies lies in their ability to estimate treatment effects in real-world settings. However, these studies commonly suffer from bias because of differences in the study groups (35). To address this issue, the treatment and comparison groups were matched on propensity scores. A propensity score is defined as the conditional probability of assignment to one treatment over another treatment given a set of pretreatment covariates (36). Propensity score matching is commonly used to minimize pretreatment differences between two groups such that the matched groups differ only on treatment assignment. Included in the propensity score calculation were more than 70 covariates, chosen on the basis of previously published literature, expert opinions, and availability from the data source (37–40). They included clinical characteristics, such as comorbid conditions and comedications, and sociodemographic characteristics, such as age, gender, and race.
Propensity scores were calculated for each individual by using pretreatment covariates. A GREEDY 5→1 matching technique was used to match nursing home patients taking paroxetine with nursing home patients taking other SSRIs (41). This technique reduces matched-pair bias caused by incomplete and inexact matching. The GREEDY 5→1 technique matches patients from the treatment group to a member of the control group on the first five digits of the propensity score. Patients who do not match are then matched on four digits and so on, reducing the number of digits matched one at a time until, if necessary, using one digit to match the remaining patients. A control patient is selected at random if more than one matched control patient is found for a patient in the treatment group.
Statistical Analysis
Statistical differences in baseline covariates across the study groups were examined by using chi square tests for categorical variables and t tests for continuous variables before and after matching. Survival analysis was performed on the matched cohort to compare the risk of dementia among users of paroxetine and users of other SSRIs. The unadjusted relationship between paroxetine and other SSRIs and the risk of dementia was evaluated by using Kaplan-Meier survival plots. A pairwise log-rank test was used to compare the survival distribution associated with paroxetine and other SSRIs. The Cox proportional hazards (PH) model was utilized to evaluate the risk of dementia associated with paroxetine versus other SSRIs.
A robust Cox model was applied by using the ID option of PROC PHREG in SAS, version 9.3 (42). Conventional Cox models assume independence of observations, whereas the robust Cox model uses a robust sandwich estimator to account for clustering within matched pairs (43). This model has been found to yield a hazard ratio (HR) with minimal bias compared with other propensity score–matched Cox models (44,45). The PH assumption for the model was checked by using the interaction term between SSRI treatment and log of time to dementia. In addition, the Schoenfeld test was conducted to examine the PH assumption. The diagnostic tests indicated that the PH assumption was not violated; thus a robust Cox hazard model was used to examine the association between SSRI use and risk of dementia. Statistical significance was set at an a priori level of .05.
Additional sensitivity analyses were conducted to evaluate the robustness of the study findings. In the first sensitivity analysis, patients were excluded if they had used memantine or cholinesterase inhibitors or had a dementia diagnosis at baseline. In addition, these antidementia medications, along with a dementia diagnosis, were used to identify dementia patients at follow-up. In the second sensitivity analysis, patients were excluded if they did not have at least one MDS assessment at baseline. Also, patients were dropped from the analysis if they had a dementia diagnosis at baseline on the basis of either an MDS assessment or CC segment indicators. In addition, MDS assessments and CC segment indicators were used to identify dementia cases at follow-up.
Results
Patient Selection and Matching
A total of 19,952 new users of SSRIs were obtained between January 2008 and December 2008 after applying inclusion and exclusion criteria (see online supplement). Of these, 1,898 patients initiated paroxetine, and 18,054 were users of other SSRIs; information about these users of SSRIs was used for calculation of propensity scores (c-statistic=.56). There were 3,796 patients in the matched cohort (N=1,898 in each group). Table 1 reports differences in baseline characteristics between the two groups before and after matching. After matching was completed, the groups were similar in terms of distribution of propensity scores (see online supplement).
| Characteristic | Unmatched cohort (N=19,952) | Matched cohort (N=3,796) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Paroxetine (N=1,898) | Other SSRIs (N=18,054) | Paroxetine (N=1,898) | Other SSRIs (N=1,898) | |||||||
| N | % | N | % | p | N | % | N | % | p | |
| Gender | .244 | .054 | ||||||||
| Female | 1,366 | 72 | 12,763 | 71 | 1,366 | 72 | 1,312 | 69 | ||
| Male | 532 | 28 | 5,291 | 29 | 532 | 28 | 586 | 31 | ||
| Age | .651 | .712 | ||||||||
| 65–84 | 1,191 | 63 | 11,424 | 63 | 1,191 | 63 | 1,202 | 63 | ||
| ≥85 | 707 | 37 | 6,630 | 37 | 707 | 37 | 696 | 37 | ||
| Race | .563 | .796 | ||||||||
| White | 1,774 | 94 | 16,975 | 94 | 1,774 | 94 | 1,784 | 94 | ||
| Nonwhite | 122 | 6 | 1,055 | 6 | 122 | 6 | 122 | 6 | ||
| Medical history | ||||||||||
| Congestive heart failure | 820 | 43 | 8,099 | 45 | .167 | 820 | 43 | 838 | 44 | .556 |
| Endocarditis | 94 | 5 | 1,132 | 6 | .023 | 94 | 5 | 129 | 7 | .016 |
| Ischemic heart disease | 1,146 | 60 | 11,168 | 62 | .207 | 1,146 | 60 | 1,209 | 64 | .035 |
| Acute myocardial infarction | 119 | 6 | 1,379 | 8 | .031 | 119 | 6 | 153 | 8 | .032 |
| Stroke or transient ischemic attack | 410 | 22 | 4,176 | 23 | .132 | 410 | 22 | 432 | 23 | .390 |
| Cardiac arrhythmia | 589 | 31 | 5,761 | 32 | .435 | 589 | 31 | 613 | 32 | .024 |
| Circulatory disorder | 651 | 34 | 6,701 | 37 | .016 | 651 | 34 | 674 | 36 | .434 |
| Thromboembolic disorder | 144 | 8 | 1,467 | 8 | .412 | 144 | 8 | 143 | 8 | .951 |
| Peripheral arterial disorder | 275 | 14 | 2,646 | 15 | .845 | 275 | 14 | 267 | 14 | .711 |
| Hypertension | 1,676 | 88 | 16,259 | 90 | .016 | 1,676 | 88 | 1,693 | 89 | .383 |
| Diabetes mellitus | 686 | 36 | 6,919 | 38 | .063 | 686 | 36 | 713 | 38 | .364 |
| Hyperlipidemia | 1,358 | 72 | 13,083 | 72 | .395 | 1,358 | 72 | 1,357 | 72 | .971 |
| Renal failure | 308 | 16 | 3,676 | 20 | <.001 | 308 | 16 | 309 | 16 | .965 |
| Other renal disease | 787 | 42 | 8,012 | 44 | .015 | 787 | 42 | 806 | 42 | .532 |
| Hip fracture | 151 | 8 | 1,449 | 8. | .915 | 151 | 8 | 149 | 8 | .904 |
| Falls | 48 | 2 | 523 | 3 | .360 | 48 | 2 | 55 | 2 | .484 |
| Osteoporosis | 602 | 32 | 5,586 | 31 | .486 | 602 | 32 | 574 | 30 | .326 |
| Rheumatoid arthritis and osteoarthritis | 1,223 | 64 | 11,943 | 66 | .134 | 1,223 | 64 | 1,252 | 66 | .323 |
| Gout and other crystal arthropathies | 82 | 4 | 770 | 4 | .909 | 82 | 4 | 88 | 5 | .638 |
| Back pain | 467 | 25 | 4,414 | 24 | .881 | 467 | 25 | 459 | 24 | .762 |
| Parkinson disease | 42 | 2 | 438 | 2 | .564 | 42 | 2 | 56 | 3 | .152 |
| Extrapyramidal syndrome | 45 | 2 | 437 | 2 | .894 | 45 | 2 | 46 | 2 | .915 |
| Fibromyalgia | 35 | 2 | 397 | 2 | .312 | 35 | 2 | 38 | 2 | .723 |
| Psychotic disorder | ||||||||||
| Anxiety | 214 | 11 | 1,924 | 11 | .408 | 214 | 11 | 192 | 10 | .248 |
| Mood disorder | 365 | 19 | 3,887 | 22 | .020 | 365 | 19 | 418 | 22 | .034 |
| Migraine | 8 | 1 | 95 | 1 | .545 | 8 | 1 | 9 | 1 | .808 |
| Schizophrenia | 54 | 3 | 513 | 3 | .993 | 54 | 3 | 54 | 3 | .539 |
| Bipolar disorder | 7 | 1 | 101 | 1 | .282 | 7 | 1 | 14 | 1 | .126 |
| Insomnia | 50 | 4 | 513 | 3 | .604 | 50 | 4 | 61 | 3 | .289 |
| Other psychiatric disorder | 279 | 15 | 2,430 | 14 | .134 | 279 | 15 | 249 | 13 | .159 |
| Liver disorder | 170 | 9 | 1,664 | 9 | .709 | 170 | 9 | 177 | 9 | .693 |
| Gastric disorder | 934 | 49 | 9,047 | 50 | .455 | 934 | 49 | 903 | 48 | .314 |
| Ulcer | 176 | 9 | 1,945 | 11 | .044 | 176 | 9 | 183 | 10 | .698 |
| Cancer | 374 | 20 | 3,475 | 19 | .631 | 374 | 20 | 369 | 19 | .838 |
| Dysphagia | 109 | 6 | 1,376 | 8 | .003 | 109 | 6 | 107 | 6 | .889 |
| Anemia | 1,160 | 61 | 11,413 | 63 | .072 | 1,160 | 61 | 1,181 | 62 | .483 |
| Asthma | 288 | 15 | 2,747 | 15 | .962 | 288 | 15 | 285 | 15 | .892 |
| Chronic obstructive pulmonary disease | 723 | 38 | 6,791 | 38 | .683 | 723 | 38 | 706 | 37 | .569 |
| Pneumonia | 274 | 14 | 2,696 | 15 | .563 | 274 | 14 | 272 | 14 | .926 |
| Benign prostatic hyperplasia | 242 | 13 | 2,507 | 14 | .172 | 242 | 13 | 272 | 14 | .155 |
| Hypothyroidism | 436 | 23 | 4,636 | 26 | .010 | 436 | 23 | 436 | 23 | .515 |
| Cataract | 1,398 | 74 | 13,679 | 76 | .042 | 1,398 | 74 | 1,438 | 76 | .135 |
| Glaucoma | 393 | 24 | 4,268 | 24 | .004 | 393 | 24 | 390 | 21 | .904 |
| Obesity | 87 | 5 | 1,080 | 6 | .014 | 87 | 5 | 88 | 5 | .938 |
| Alcohol and drug dependence | 58 | 3 | 557 | 3 | .944 | 58 | 3 | 57 | 3 | .925 |
| Use of other drugs | ||||||||||
| Anti-infective agent | 1,389 | 73 | 13,368 | 74 | .416 | 1,389 | 73 | 1,396 | 74 | .797 |
| Endocrine and metabolic drug | 1,227 | 65 | 11,934 | 66 | .203 | 1,227 | 65 | 1,230 | 65 | .918 |
| Cardiovascular agent | 1,597 | 84 | 15,671 | 87 | .001 | 1,597 | 84 | 1,600 | 84 | .894 |
| Antihyperlipidemic drug | 772 | 41 | 7,953 | 44 | .005 | 772 | 41 | 854 | 45 | .007 |
| Respiratory agent | 659 | 35 | 6,263 | 35 | .979 | 659 | 35 | 677 | 36 | .541 |
| Antihistamine or other cold remedy | 411 | 22 | 3,847 | 21 | .726 | 411 | 22 | 403 | 21 | .752 |
| Gastrointestinal agent | 433 | 23 | 4,242 | 24 | .279 | 433 | 23 | 439 | 23 | .817 |
| Genitourinary product | 213 | 11 | 2,179 | 12 | .279 | 213 | 11 | 215 | 11 | .918 |
| Antianxiety agent | 89 | 5 | 850 | 5 | .970 | 89 | 5 | 99 | 5 | .454 |
| Antipsychotic | 161 | 8 | 1,646 | 9 | .360 | 161 | 8 | 153 | 8 | .637 |
| Hypnotic | 219 | 12 | 2,159 | 12 | .591 | 219 | 12 | 193 | 10 | .175 |
| Stimulant, antiobesity agent, anorexiant | 66 | 4 | 462 | 3 | .018 | 66 | 4 | 65 | 3 | .929 |
| Other psychotherapeutic agent | 34 | 2 | 356 | 2 | .589 | 34 | 2 | 34 | 2 | .549 |
| Anticonvulsant | 262 | 14 | 2,641 | 15 | .333 | 262 | 14 | 275 | 14 | .545 |
| Antiparkinsonian | 89 | 5 | 936 | 5 | .353 | 89 | 5 | 104 | 5 | .268 |
| Analgesic or anti-inflammatory | 1131 | 60 | 10,880 | 60 | .568 | 1.131 | 60 | 1,125 | 59 | .843 |
| Musculoskeletal agent | 443 | 23 | 4,513 | 25 | .112 | 443 | 23 | 465 | 24 | .403 |
| Nutritional product | 497 | 26 | 5,100 | 28 | .057 | 497 | 26 | 508 | 27 | .686 |
| Hematological agent | 627 | 33 | 6,156 | 34 | .352 | 627 | 33 | 692 | 36 | .027 |
| Topical product | 862 | 45 | 8,549 | 47 | .108 | 862 | 45 | 885 | 47 | .454 |
| Central acetylcholinesterase inhibitor | 20 | 1 | 280 | 2 | .091 | 20 | 1 | 32 | 2 | .094 |
| Alcohol and drug dependence agent | 11 | 1 | 204 | 1 | .027 | 11 | .1 | 11 | .1 | .584 |
| Antineoplastic agent | 106 | 6 | 942 | 5 | .495 | 106 | 6 | 106 | 6 | .944 |
TABLE 1. Characteristics of elderly nursing home patients during the 12 months prior to initiating use of paroxetine or other selective serotonin reuptake inhibitors (SSRIs)
Risk of Dementia
There were 306 incident cases of dementia in the matched cohort during the follow-up period. The incidence dementia rate was 7.5% for the paroxetine users and 8.6% for users of other SSRIs, a nonsignificant difference. Figure 1 shows Kaplan-Meier survival curves evaluating the risk of dementia among elderly patients with depression who used paroxetine or other SSRI antidepressants. The graph demonstrates that there was no difference across SSRI use and risk of dementia. The results from the Schoenfeld test indicated that the PH assumption was met (p=.06).
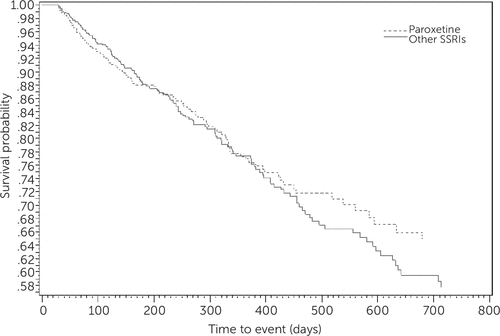
FIGURE 1. Kaplan Meier curve representing the association between use of paroxetine and other selective serotonin reuptake inhibitors (SSRIs) and risk of dementiaa
a Survival probability indicates the percentage of patients who did not develop dementia. There was no association between risk of dementia and use of paroxetine or other SSRIs.
Table 2 presents results from the robust Cox PH model. The analysis revealed that there was no significant difference in risk of dementia between paroxetine users and users of other SSRIs. An additional robust Cox PH regression was performed after controlling for variables that were significant after propensity score matching. The findings remained unchanged in this model. There was no significant difference in the risk of dementia between paroxetine users and users of other SSRIs after the model controlled for gender, endocarditis, ischemic heart disease, acute myocardial infarction, arrhythmia, mood disorder, antihyperlipidemic drugs, and hematological agents.
| Analysis | Paroxetine | Other SSRIs | HRa | 95% CI | p |
|---|---|---|---|---|---|
| Propensity score–matched PH modelb | 1,898 | 1,898 | .99 | .79–1.23 | .896 |
| Adjusted PH modelc | 1,898 | 1,898 | .96 | .77–1.21 | .755 |
| First sensitivity analysis | 1,859 | 1,859 | .98 | .79–1.22 | .862 |
| Second sensitivity analysis | 804 | 804 | 1.18 | .84–1.66 | .337 |
TABLE 2. Risk of dementia among elderly patients using paroxetine versus other selective serotonin reuptake inhibitors (SSRIs)
Additional Sensitivity Analysis
Multiple sensitivity analyses were performed to evaluate the robustness of the study findings (Table 2). In the first sensitivity analysis, measurement of dementia on the basis of diagnosis or medications yielded 19,488 patients. Of these, 1,859 initiated paroxetine and 17,589 were users of other SSRIs. There were 3,718 patients in the matched cohort (N=1,859 in each group). In this analysis, there was no difference in the risk of dementia among the paroxetine users. Results from this sensitivity analysis supported the study findings.
In the second sensitivity analysis, patients were excluded if they did not have at least one MDS assessment at baseline, which yielded 9,505 patients. Of these, 804 initiated paroxetine and 8,701 were users of other SSRIs. There were 1,608 patients in the matched cohort (N=804 in each group). Consistent with the main findings, there was no statistically significant difference in the risk of dementia among the paroxetine users compared with users of other SSRIs.
Discussion
Prescribing medications with significant anticholinergic effects for the elderly population is often considered potentially inappropriate (46–49). The Beers’ (50,51) criteria and their updates (52) have identified several medications with anticholinergic properties to avoid when prescribing to the elderly in general and to persons with impaired cognition. Avoiding drugs with strong anticholinergic properties is also a quality-of-care indicator in Accessing Care of Vulnerable Elders (ACOVE), a set of quality measures specifically developed for the vulnerable elderly (53). Furthermore, the Healthcare Effectiveness Data and Information Set has created quality-of-care measures for use of anticholinergic medications among patients with dementia, given that these medications can lead to worsened cognitive function among patients with dementia. Among the SSRIs, paroxetine is highly anticholinergic and can lead to adverse cognitive effects. This is the first study, to our knowledge, to establish that paroxetine, although highly anticholinergic, does not increase the risk of dementia among elderly nursing home residents with depression compared with other SSRIs.
Paroxetine has been found to have higher affinity for muscarinic acetylcholine receptors compared with other SSRIs (20,21). Thus paroxetine-induced anticholinergic side effects can increase the risk of dementia, especially among elderly persons with depression, who are already at risk of cognitive impairment. Previous studies have shown that anticholinergic use is associated with risk of cognitive decline and dementia (12–14), but the current study findings suggest that this risk is no higher for paroxetine than for other SSRIs. These results indicate that pharmacological differences between paroxetine and other SSRIs do not translate into significant differences with respect to risk of dementia. Unlike previous studies that examined all anticholinergics, this study focused on persons who were treated with SSRIs to evaluate the comparative risk and thus minimized indication bias.
The study findings do not suggest that there is no association between use of paroxetine and risk of cognitive decline and dementia; rather these findings indicate that paroxetine and other SSRIs confer similar risk. Among new users of SSRIs, around 9% initiated treatment with paroxetine. This utilization pattern is consistent with the findings from previous studies (23–25). Given the frequent use of paroxetine and other SSRIs in nursing homes, there is a need for future studies to better understand the short-term cognitive effects and long-term dementia risk of paroxetine compared with other SSRIs. Although paroxetine and other SSRIs have comparable effectiveness profiles (17), they are not same; thus prescribers need to evaluate baseline cognition function and anticholinergic burden before selecting an antidepressant for the treatment of depression among elderly patients.
This study evaluated comparative safety of paroxetine and other SSRIs in the vulnerable population of nursing home residents with depression by implementing a new-user design. Class-specific analyses using a propensity score–matching technique helped to control for indication and selection bias. In addition, this study had several advantages compared with randomized trials, such as a large sample size of elderly patients, a long follow-up period, and real-world data.
Prescription claims are valid and reliable sources for gathering medication-related information (54,55). However, this study had some limitations. The diseases and outcome measurements were based on diagnostic data in medical claims. Long-term exposure to SSRIs was ascertained by using Part D claims data. Use of medications as a component of Part A bundled payments for short postacute nursing home stays was not captured. Also, the pharmacy claims captured only dispensing data and not actual use by patients. This study used Medicare files, which are secondary data and thus have limitations due to miscoding and undercoding (56). Other factors related to the risk of dementia, such as apolipoprotein E-e4, other genetic factors, diet, physical activity, stress, smoking, and alcohol consumption (11), were not available in the database and could not be analyzed in the study. Propensity score approaches control only for observed confounders. There is a chance that unobserved confounding can affect study findings. However, multiple sensitivity analyses supported the main findings.
Conclusions
This retrospective study using propensity score matching found no difference in the risk of dementia associated with paroxetine compared with other SSRIs among elderly nursing home residents with depression. Although paroxetine has stronger anticholinergic properties compared with other SSRIs, the current study suggests that pharmacological differences between paroxetine and other SSRIs do not translate into significant differences with respect to risk of dementia. Given the frequent use of paroxetine, there is a need for future studies to better understand differences in short-term cognitive effects and long-term dementia risk associated with the use of paroxetine and other SSRIs among elderly persons with depression.
1 : Dementia risk prediction: are we there yet? Clinics in Geriatric Medicine 26:113–123, 2010Crossref, Medline, Google Scholar
2 : Dissecting dementia, depression, and drug effects in older adults. Journal of Psychosocial Nursing and Mental Health Services 48:39–47, 2010Crossref, Medline, Google Scholar
3 : Geriatric depression and cognitive impairment. Psychological Medicine 38:163–175, 2008Crossref, Medline, Google Scholar
4 : Forecasting the global burden of Alzheimer's disease: Alzheimer’s and dementia. Journal of the Alzheimer's Association 3:186–191, 2007Crossref, Medline, Google Scholar
5 : Alzheimer's disease facts and figures: Alzheimer’s and dementia. Journal of the Alzheimer's Association 7:208–244, 2011Crossref, Medline, Google Scholar
6 : Promising strategies for the prevention of dementia. Archives of Neurology 66:1210–1215, 2009Crossref, Medline, Google Scholar
7 : The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatrica Scandinavica 76:465–479, 1987Crossref, Medline, Google Scholar
8 : Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry 63:530–538, 2006Crossref, Medline, Google Scholar
9 : The incidence of dementia: a meta-analysis. Neurology 51:728–733, 1998Crossref, Medline, Google Scholar
10 : A quantitative review of cognitive deficits in depression and Alzheimer-type dementia. Journal of the International Neuropsychological Society 3:631–651, 1997Crossref, Medline, Google Scholar
11 Williams JW, Plassman BL, Burke J, et al: Preventing Alzheimer’s Disease and Cognitive Decline. Evidence Report/Technology Assessment no 193. Rockville, Md, Agency for Healthcare Research and Quality, April 2010Google Scholar
12 : Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology 75:152–159, 2010Crossref, Medline, Google Scholar
13 : Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Archives of Internal Medicine 169:1317–1324, 2009Crossref, Medline, Google Scholar
14 : The cognitive impact of anticholinergics: a clinical review. Clinical Interventions in Aging 4:225–233, 2009Medline, Google Scholar
15 : Pharmacotherapy of depressive disorders in older patients. Postgraduate Medicine Spec No Pharmacotherapy:1–86, 2001Google Scholar
16 Salzman C, Small GW: Treatment of depression with new and atypical antidepressants; in Clinical Geriatric Psychopharmacology. Edited by Salzman C. Philadelphia, Lippincott, Williams and Wilkins, 2005Google Scholar
17 : Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Annals of Internal Medicine 155:772–785, 2011Crossref, Medline, Google Scholar
18 : Comparative risk for harms of second-generation antidepressants: a systematic review and meta-analysis. Drug Safety 31:851–865, 2008Crossref, Medline, Google Scholar
19 : Selective serotonin reuptake inhibitors in affective disorders—I. basic pharmacology. Journal of Psychopharmacology 12(suppl B):S5–S20, 1998Crossref, Medline, Google Scholar
20 : A comparative review of escitalopram, paroxetine, and sertraline: are they all alike? International Clinical Psychopharmacology 29:185–196, 2014Crossref, Medline, Google Scholar
21 : Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. Journal of Pharmacology and Experimental Therapeutics 283:1305–1322, 1997Medline, Google Scholar
22 American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society 60:616–631, 2012Crossref, Medline, Google Scholar
23 : Prevalence and predictors of antidepressant prescribing in nursing home residents in the United States. American Journal of Geriatric Pharmacotherapy 9:109–119, 2011Crossref, Medline, Google Scholar
24 : Prevalence of anticholinergic medication use among elderly nursing home residents with depression. Value in Health 17:A211, 2014Crossref, Google Scholar
25 : Risk of dementia associated with the use of paroxetine among the elderly nursing home patients with depression. Value in Health 17:A209, 2014Crossref, Google Scholar
26 : Assessing medication exposures and outcomes in the frail elderly: assessing research challenges in nursing home pharmacotherapy. Medical Care 48(suppl):S23–S31, 2010Crossref, Medline, Google Scholar
27 : Medicare Part D data: a valuable tool for pharmacoepidemiology and pharmacoeconomic research. American Journal of Geriatric Pharmacotherapy 8:483–484, 2010Crossref, Medline, Google Scholar
28 Identifiable Data Files. Baltimore, Centers for Medicare and Medicaid Services. Available at www.cms.hhs.gov/IdentifiableDataFiles/02_StandardAnalyticalFiles.asp. Accessed Oct 30, 2014Google Scholar
29 : Receipt of monitoring of diabetes mellitus in older adults with comorbid dementia. Journal of the American Geriatrics Society 60:644–651, 2012Crossref, Medline, Google Scholar
30 : Prevalence of multiple chronic conditions in the United States’ Medicare population. Health and Quality of Life Outcomes 7:82:1–11, 2009Crossref, Medline, Google Scholar
31 : Using Medicare data for comparative effectiveness research: opportunities and challenges. American Journal of Managed Care 17:488–496, 2011Medline, Google Scholar
32 : Methods for evaluating patient adherence to antidepressant therapy: a real-world comparison of adherence and economic outcomes. Medical Care 44:300–303, 2006Crossref, Medline, Google Scholar
33 : Medication compliance and persistence: terminology and definitions. Value in Health 11:44–47, 2008Crossref, Medline, Google Scholar
34 : Adherence and persistence with branded antidepressants and generic SSRIs among managed care patients with major depressive disorder. ClinicoEconomics and Outcomes Research 3:63–72, 2011Medline, Google Scholar
35 : Too much ado about propensity score models? Comparing methods of propensity score matching. Value in Health 9:377–385, 2006Crossref, Medline, Google Scholar
36 : Variable selection for propensity score models. American Journal of Epidemiology 163:1149–1156, 2006Crossref, Medline, Google Scholar
37 : Risk of death in dual-eligible nursing home residents using typical or atypical antipsychotic agents. Medical Care 50:961–969, 2012Crossref, Medline, Google Scholar
38 : Risk of falls and fractures in older adults using atypical antipsychotic agents: a propensity score-adjusted, retrospective cohort study. American Journal of Geriatric Pharmacotherapy 10:83–94, 2012Crossref, Medline, Google Scholar
39 : Comparative risk of cerebrovascular adverse events in community-dwelling older adults using risperidone, olanzapine and quetiapine: a multiple propensity score–adjusted retrospective cohort study. Drugs and Aging 29:807–817, 2012Crossref, Medline, Google Scholar
40 : Risk of hospitalization and use of first- versus second-generation antipsychotics among nursing home residents. Psychiatric Services 65:781–788, 2014Link, Google Scholar
41 Parson LS: Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. Edmonds, Wash, Ovation Research Group, 2001. Available at www2.sas.com/proceedings/sugi26/p214-26.pdfGoogle Scholar
42 SAS 9.3 Guide to Software Updates. Cary, NC, SAS Institute, Inc, 2011Google Scholar
43 : The robust inference for the Cox proportional hazards model. Journal of the American Statistical Association 84:1074–1078, 1989Crossref, Google Scholar
44 : The performance of different propensity score methods for estimating marginal hazard ratios. Statistics in Medicine 32:2837–2849, 2013Crossref, Medline, Google Scholar
45 : Propensity score applied to survival data analysis through proportional hazards models: a Monte Carlo study. Pharmaceutical Statistics 11:222–229, 2012Crossref, Medline, Google Scholar
46 : Anticholinergic effects of medication in elderly patients. Journal of Clinical Psychiatry 62(suppl 21):11–14, 2001Medline, Google Scholar
47 : The problems of anticholinergic adverse effects in older patients. Drugs and Aging 3:335–348, 1993Crossref, Medline, Google Scholar
48 : Anticholinergic medications: use among older adults with memory problems. Journal of Gerontological Nursing 33:21–29, 2007Crossref, Medline, Google Scholar
49 : A pharmacoepidemiological evaluation of anticholinergic prescribing patterns in the elderly. Pharmacoepidemiology and Drug Safety 5:155–164, 1996Crossref, Medline, Google Scholar
50 : Explicit criteria for determining inappropriate medication use in nursing home residents. Archives of Internal Medicine 151:1825–1832, 1991Crossref, Medline, Google Scholar
51 : Explicit criteria for determining potentially inappropriate medication use by the elderly: an update. Archives of Internal Medicine 157:1531–1536, 1997Crossref, Medline, Google Scholar
52 : Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Archives of Internal Medicine 163:2716–2724, 2003Crossref, Medline, Google Scholar
53 : Quality indicators for medication use in vulnerable elders. Journal of the American Geriatrics Society 55(suppl 2):S373–S382, 2007Crossref, Medline, Google Scholar
54 : The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Québec. Journal of Clinical Epidemiology 48:999–1009, 1995Crossref, Medline, Google Scholar
55 : Comparison of medical records and prescription claims files in documenting prescription medication therapy. Journal of Pharmacoepidemiology 5:3–16, 1996Crossref, Google Scholar
56 : How late-life depression affects cognition: neural mechanisms. Current Psychiatry Reports 12:34–38, 2010Crossref, Medline, Google Scholar



