Improvement in Body Image, Perceived Health, and Health-Related Self-Efficacy Among People With Serious Mental Illness: The STRIDE Study
Abstract
Objective:
The authors examined secondary outcomes of STRIDE, a randomized controlled trial that tested a weight-loss and lifestyle intervention for individuals taking antipsychotic medications.
Methods:
Hierarchical linear regression was used to explore the effects of the intervention and weight change at follow-up (six, 12, and 24 months) on body image, perceived health, and health-related self-efficacy.
Results:
Participants were 200 adults who were overweight and taking antipsychotic agents. Weight change × study arm interaction was associated with significant improvement in body image from baseline to six months. From baseline to 12 months, body image scores of intervention participants improved by 1.7 points more compared with scores of control participants; greater weight loss was associated with more improvement. Between baseline and 24 months, greater weight loss was associated with improvements in body image, perceived health, and health-related self-efficacy.
Conclusions:
Participation in STRIDE improved body image, and losing weight improved perceived health and health-related self-efficacy.
Individuals with serious mental illnesses have a greatly reduced life expectancy (1–8) because of cardiometabolic diseases and their associated risks, prompting the development of lifestyle-modification interventions specifically adapted for this population. These include programs focused on smoking cessation, behavioral weight-loss interventions, and comprehensive lifestyle change (9–15).
We conducted a randomized controlled trial of a weight-loss and lifestyle-change intervention for individuals with serious mental illnesses who were taking antipsychotic medications. The study, called STRIDE, used an adapted version of the PREMIER comprehensive lifestyle intervention (16,17) with DASH dietary pattern (18). The trial showed that participants lost significantly more weight compared with control participants and reduced their diabetes risk, while other indicators of cardiovascular risks also showed trends toward improvement (15).
In this article, we report results of analyses of secondary outcomes, including the effects of the intervention and change in body weight on body image, perceived health, and health-related self-efficacy. Each of these outcomes is directly targeted by the intervention. We hypothesized that the intervention group would show greater improvement in these secondary outcomes. In addition, we hypothesized that the amount of weight loss would moderate the effect on the secondary outcomes. A better understanding of how lifestyle interventions and weight loss affect these domains may suggest methods and targets that could optimize lifestyle interventions.
Methods
STRIDE was implemented in three publicly funded community mental health centers and a not-for-profit integrated health plan. The data collection period was July 2009 through October 2013. We recruited adults (age ≥18) who were overweight or obese (BMI ≥27 kg/m2), received services at one of the study sites, and had been taking antipsychotic medications for at least a month. Case managers promoted the study, and letters were sent to potentially eligible recruits; study staff followed up by phone. Potential recruits were excluded if they were pregnant, were planning a pregnancy during the study period, or were breastfeeding; had an inpatient psychiatric hospitalization within the prior month (although we allowed deferred participation); had a history of, or plans for, bariatric surgery; had a history of cancer in the two years prior to enrollment; had a heart attack or stroke in the past six months; or had moderate to severe cognitive impairment that could interfere with their ability to provide informed consent or participate in the group intervention.
To ensure even distribution by gender and BMI (low, 27–34.9 kg/m2, versus high, ≥35 kg/m2) across study arms, all enrolled participants were randomly assigned to the intervention or control group by using a stratified randomized block design. The Kaiser Permanente Northwest Institutional Review Board reviewed, approved, and monitored all study sites and procedures. All participants provided written informed consent prior to enrolling in the trial.
The intervention consisted of two six-month phases, an intensive phase that involved weekly group meetings and a maintenance phase. The goal of the intensive phase was weight loss of 4.5–6.8 kg, or ten to 15 pounds. The maintenance phase, which involved monthly group meetings and individual telephone consults as needed, was designed to help participants maintain behavior changes and weight loss.
The intervention focused on improving diet (increasing fruit, vegetable, and low-fat dairy consumption and reducing fat consumption), limiting calories and portions, and increasing moderate-intensity exercise (including 20 minutes of exercise during the intervention sessions). Cognitive-behavioral therapy tools (such as goal setting, self-monitoring, problem solving, and cognitive restructuring) were used throughout the intervention. Participants were encouraged to set gradual and realistic weight-loss goals, self-monitor diet and exercise, and check their weight weekly to monitor progress. Achievements and challenges were discussed during group check-ins, participants were encouraged to help one another brainstorm ways to overcome obstacles, and group leaders facilitated reframing setbacks as opportunities for improvement.
In intent-to-treat analyses, intervention participants lost more weight than control participants from baseline to six months (4.4 kg, 95% confidence interval [CI]=–6.96 to –1.78 kg) and from baseline to 12 months (2.6 kg, CI=–5.14 to –.07 kg) (15). In addition, at 12 months, there was a significant time × group interaction related to fasting glucose levels, which increased among control participants but declined among intervention participants. Further, fewer participants in the intervention group reported medical hospitalizations compared with the control group (7% and 19%, respectively; χ2=6.66, df=1, p=.01) (15).
Details of the study design and rationale (19,20) and primary results of the trial at six, 12, and 24 months have been previously reported (15,21). The intervention manual and related materials are available online at www.kpchr.org/stridepublic.
Measures
Body image.
Participants completed the Body Weight, Image and Self-Esteem Evaluation (B-WISE) questionnaire (22). This 12-item measure is designed for use in psychiatric populations to quantify the psychosocial impact of weight changes associated with psychotropic drug use.
General health.
We used the five-item SF-36v2 general health subscale as an indicator of health status; it is reliable and valid for people with serious mental illnesses and multiple general health conditions (23,24) and has been nationally normed.
Health-related self-efficacy.
The 13-item Patient Activation Measure (PAM) (25) measures activation and engagement in personal health matters in four developmental dimensions: believing in taking an active role to manage one’s self-care; having the confidence, knowledge, and skills to take action; taking action to maintain or improve one’s health; and staying the course under stress.
Statistical Analyses
All statistical procedures were performed using Stata 13 (26). We used hierarchical multiple linear regression analyses to explore effects of the intervention and change in weight on body image, general health, and health-related self-efficacy at each follow-up assessment (six, 12, and 24 months), computing separate analyses for each outcome and follow-up period. The first block included demographic variables, site, and diagnostic group. The second block included an indicator of study group (intervention versus usual care), and the third block included change in weight from baseline. The fourth and final block included an intervention × weight change product term. This term allowed us to assess whether the relationship between weight change and the secondary outcome differed by study group (moderator or interaction effect). Support for a moderation effect was indicated if the addition of the interaction term was associated with a significant change in the variance explained (Δ R2) in the model. Significant interactions were graphed to interpret the nature of the effect.
Given the small sample size, similar rates of attrition between the control and intervention groups, and reasonable follow-up rate at 24 months, we did not use advanced techniques for handling missing values, such as multiple imputation. Instead, we based the analyses on participants with complete data at each set of time points (listwise approach). All unstandardized coefficients reported were from the final main-effects model (the third block), unless the interaction was significant, in which case the coefficients from the fourth block (which included all blocks) are reported. We checked for the presence of multicollinearity by calculating the variance inflation factors (VIFs) for each model, using the common threshold of values below ten as acceptable.
Results
Participants were 200 adults, of whom 72% (N=144) were female, 88% (N=174) white, 6% (N=12) African American, 4% (N=8) Native American, 3% (N=6) Asian, and 2% (N=4) of Hispanic ethnicity, with a mean±SD age of 47.2±10.6 and a mean BMI of 38.3±8.3 kg/m2. All were taking antipsychotic agents for at least a month prior to enrollment; 58 (29%) had a schizophrenia spectrum diagnosis, 138 (69%) had a diagnosis of bipolar disorder or affective psychosis, and 4 (2%) were diagnosed as having posttraumatic stress disorder.
Randomization resulted in assignment of 96 participants to usual care and 104 to the intervention arm of the study. Table 1 presents the baseline values of each outcome by arm; there was no evidence of imbalance between the arms. We collected data from 91% of the sample (N=181) at six months, 85% (N=170) at 12 months, and 82% (N=164) at 24 months.
| Control (N=96) | Intervention (N=104) | ||||
|---|---|---|---|---|---|
| Measure | M | SD | M | SD | pa |
| Body imageb | 21.8 | 3.7 | 21.9 | 3.5 | .84 |
| Perceived healthc | 41.3 | 8.8 | 42.8 | 10.9 | .31 |
| Health-related self-efficacyd | 61.8 | 14.4 | 63.0 | 17.5 | .61 |
TABLE 1. Baseline characteristics of 200 participants in a weight-loss and lifestyle intervention, by study arm
Table 2 presents the results of the hierarchical regression analyses for each of the outcomes for each pair of time points. The weight change × study arm interaction from baseline to six months had a significant effect on body image (Δ R2=.05, p=.002); the simple effects are displayed in Figure 1. In the intervention group, individuals who lost more weight showed greater improvement in body image. The same pattern was observed in the control group; however, the relationship between change in body image and weight change was smaller.
| Outcome and follow-up | ba | p | ∆ R2b |
|---|---|---|---|
| Body image | |||
| 6 months | |||
| Study arm | .59 | .318 | .043 |
| Weight change | –.03 | .442 | .055 |
| Weight change × study arm interaction | –.21 | .002 | .053 |
| 12 months | |||
| Study armc | 1.66 | .009 | .068 |
| Weight changec | –.20 | <.001 | .141 |
| Weight change × study arm interaction | –.04 | .581 | .001 |
| 24 months | |||
| Study armc | .60 | .375 | .012 |
| Weight changec | –.18 | <.001 | .161 |
| Weight change × study arm interaction | .09 | .172 | .010 |
| Perceived health | |||
| 6 months | |||
| Study armc | .50 | .726 | .001 |
| Weight changec | –.03 | .697 | .001 |
| Weight change × study arm interaction | –.04 | .792 | <.001 |
| 12 months | |||
| Study armc | .63 | .676 | .003 |
| Weight changec | –.11 | .218 | .009 |
| Weight change × study arm interaction | .14 | .437 | .004 |
| 24 months | |||
| Study armc | .37 | .805 | .002 |
| Weight changec | –.17 | .024 | .032 |
| Weight change × study arm interaction | .04 | .774 | .001 |
| Health-related self-efficacy | |||
| 6 months | |||
| Study armc | 1.40 | .599 | .002 |
| Weight changec | –.02 | .920 | <.001 |
| Weight change × study arm interaction | –.18 | .554 | .002 |
| 12 months | |||
| Study armc | 4.32 | .122 | .020 |
| Weight changec | –.19 | .237 | .009 |
| Weight change × study arm interaction | .45 | .180 | .011 |
| 24 months | |||
| Study armc | .72 | .799 | .002 |
| Weight change | –.47 | .001 | .065 |
| Weight change × study arm interaction | .33 | .241 | .008 |
TABLE 2. Effects of a weight-loss and lifestyle intervention and weight change over time on body image, perceived health, and health-related self-efficacy
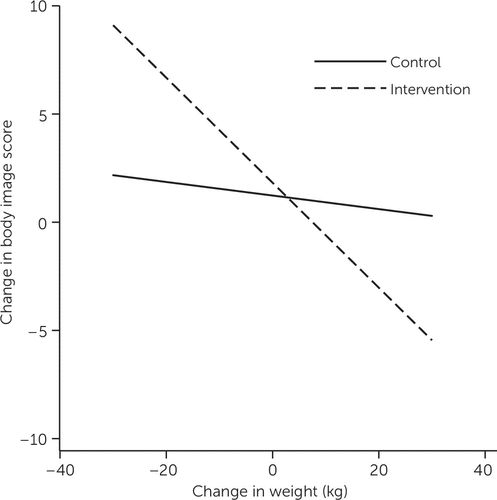
FIGURE 1. Effects of weight change × study arm interaction on body image at six-month follow-upa
a Individuals in the intervention group who lost more weight showed greater improvement in body image (p=.002), as measured by the Body Weight, Image and Self-Esteem Evaluation (possible scores range from 12 to 36, with higher scores indicating better psychosocial adjustment to weight gain).
Between baseline and 12 months, only the main effects of arm and weight change were associated with significant changes in body image. From baseline to 12 months, mean scores on the B-WISE improved by 1.7 points more for participants in the intervention group than for the control group (p=.009). Also, participants with greater weight loss at 12 months showed more improvement in body image (b=–.20, p<.001). At 24 months, only the main effect of weight change was significant—participants who lost more weight had greater improvements in body image (b=–.20, p<.001).
Between baseline and 24 months, after the analyses controlled for all other variables, greater weight loss was associated with a perception of improved health (b=–.20, p=.024) and health-related self-efficacy (b=–.50, p=.001). There was no evidence for multicollinearity issues in any model, with the highest VIF being 2.9.
Discussion
After six months, participants in the STRIDE intervention who lost weight experienced improved body image compared with control participants who lost weight. This finding suggests that the intervention, above and beyond its effects on weight loss, had a positive influence on participants’ self-perception. Put another way, if two participants, one in each arm of the study, lost the same amount of weight, the one in the intervention arm would experience a greater improvement in body image, compared with the control participant, by virtue of having received the intervention.
There are a few ways in which STRIDE participation may have positively affected body image. First, STRIDE group leaders explicitly and repeatedly encouraged participants to have realistic expectations about weight and weight loss, which may have led to greater acceptance of body shape and size and greater tolerance of gradual weight loss progress. Second, cognitive inflexibility—a common, self-defeating obstacle to weight loss—was widespread among participants in both the control and intervention arms (27). Group leaders conducted a session focused on reframing negative self-talk and used cognitive-behavioral techniques throughout the intervention to help participants overcome negative thought patterns. Participants were consistently reinforced for even modest successes, and perceived failures were discussed by the group and reframed as opportunities for improvement.
Finally, we suspect that being in a group of people with similar challenges facilitated more realistic self-evaluation. Like obesity, mental illnesses are stigmatizing (28,29), and persons with both conditions may be at greater risk of experiencing stigma’s negative consequences, including body dissatisfaction, low self-esteem, and, for women, internalizing the thin ideal. Social comparison is a powerful force and is usually considered a negative influence if the comparison is with idealized media images of what bodies should look like (30). In a supportive context, however, social comparison may allow one to see how bodies actually look and to generate cognitive dissonance that results in a change in self-perception. For example, if a person concludes that he or she cannot lose weight because of a lack of willpower and then participates in a group in which people appear to be working hard to lose weight but are losing only a modest amount, he or she must reconcile beliefs about willpower in the face of disconfirming evidence. It may be that STRIDE participants had opportunities to reevaluate a negative self-image in light of their experiences in the groups.
Improving body image is an important outcome because body dissatisfaction is correlated with physical and mental problems (31). For men, body dissatisfaction is associated with poor psychological adjustment, eating disorders, compulsive exercising, and steroid use (32). For women, body dissatisfaction is related to decreased pleasant feelings, increased negative feelings, depression, disordered eating and associated pathology, decreased self-care, and decreased quality of life (33,34). To our knowledge, this is the first study to investigate the effects of modest weight loss or participation in a lifestyle-change program on body image enhancement among overweight individuals with serious mental illnesses.
At 24 months, greater weight loss was associated with greater increases in self-image, but the effect of the intervention arm was no longer significant. This suggests that the intervention’s effects on body image enhancement diminished after group support was discontinued. In our study of barriers and facilitators of weight loss among STRIDE participants (27), participants described backsliding after the groups stopped meeting weekly and called for an extension of group support.
Finally, at the 24-month follow-up, greater weight loss was also associated with improvements in perceived health and health-related self-efficacy. We are encouraged by these results because improving health and facilitating health-related self-efficacy are implicit goals of lifestyle-change programs. We find it interesting, however, that these outcomes were not realized until the 24-month follow-up, although general medical hospitalizations were significantly reduced in the intervention group at both 12 (15) and 24 months (21). This finding suggests that it may take some time for people to feel the effects of weight loss on their perceived health and to gain confidence that they can manage and affect their health outcomes. This possibility presents an opportunity to improve STRIDE by adding an introductory phase that imparts knowledge about health and health-related risks; directly connects actions to anticipated results; and focuses on developing positive outcome expectancies, optimistic beliefs about capacity to overcome barriers, motivation and intention to change health-related behaviors, and conviction and skills to get back on track after lapses. Improvements in body image may have been the result of coaching and feedback that explicitly connected the participants’ efforts and accomplishments within the context of realistic expectations. In the same way, priming participants to expect health benefits from their efforts and helping them to see linkages between their actions and health-related consequences could hasten their recognition of improved health and assist them to feel more effective at managing their health.
A limitation of this work concerned the lack of representation of men (28%), racial-ethnic minority group members (13%), and younger people (mean age=47.2) in the study sample. Our results may not be generalizable to these groups. Future research should explore methods of involving these groups in lifestyle-change programs.
Conclusions
This analysis of secondary outcomes from the STRIDE study indicated that, in addition to facilitating weight loss and decreasing diabetes risk, participation in STRIDE effectively improved body image and that losing weight improved perceived health and health-related self-efficacy. Providers should be encouraged to promote weight-loss and lifestyle-change efforts among their patients with serious mental illnesses, and medical and mental health systems should consider offering supportive programs of this type as part of recovery-focused care.
1 : Are VA patients with serious mental illness dying younger? Psychiatric Services 60:589, 2009Link, Google Scholar
2 : The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ 346:f2539, 2013Crossref, Medline, Google Scholar
3 : A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of General Psychiatry 64:1123–1131, 2007Crossref, Medline, Google Scholar
4 Parks J, Svendsen D, Singer P, et al (eds): Morbidity and Mortality in People With Serious Mental Illness. Alexandria, Va, National Association of State Mental Health Program Directors Medical Directors, 2007Google Scholar
5 : Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disorders 6:368–373, 2004Crossref, Medline, Google Scholar
6 : Psychiatric and medical comorbidities of bipolar disorder. Psychosomatic Medicine 67:1–8, 2005Crossref, Medline, Google Scholar
7 : A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophrenia Research 80:45–53, 2005Crossref, Medline, Google Scholar
8 : Comparative mortality risk in adult patients with schizophrenia, depression, bipolar disorder, anxiety disorders, and attention-deficit/hyperactivity disorder participating in psychopharmacology clinical trials. JAMA Psychiatry 70:1091–1099, 2013Crossref, Medline, Google Scholar
9 : Clinically significant improved fitness and weight loss among overweight persons with serious mental illness. Psychiatric Services 64:729–736, 2013Link, Google Scholar
10 : A behavioral weight-loss intervention in persons with serious mental illness. New England Journal of Medicine 368:1594–1602, 2013Crossref, Medline, Google Scholar
11 : Shared decision making and behavioral support interventions for people with severe mental illness and tobacco dependence. Journal of Dual Diagnosis 8:99–103, 2012Crossref, Google Scholar
12 : Comparison of two intensities of tobacco dependence counseling in schizophrenia and schizoaffective disorder. Journal of Substance Abuse Treatment 38:384–393, 2010Crossref, Medline, Google Scholar
13 : Weight loss intervention for people with serious mental illness: a randomized controlled trial of the RENEW program. Psychiatric Services 62:800–802, 2011Link, Google Scholar
14 : Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA 311:145–154, 2014Crossref, Medline, Google Scholar
15 : the STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. American Journal of Psychiatry 172:71–81, 2015Link, Google Scholar
16 : Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 289:2083–2093, 2003Medline, Google Scholar
17 : PREMIER—a trial of lifestyle interventions for blood pressure control: intervention design and rationale. Health Promotion Practice 9:271–280, 2008Crossref, Medline, Google Scholar
18 : The effect of the PREMIER interventions on insulin sensitivity. Diabetes Care 27:340–347, 2004Crossref, Medline, Google Scholar
19 : Delivering a lifestyle and weight loss intervention to individuals in real-world mental health settings: lessons and opportunities. Translational Behavioral Medicine 1:406–415, 2011Crossref, Medline, Google Scholar
20 : STRIDE: a randomized trial of a lifestyle intervention to promote weight loss among individuals taking antipsychotic medications. BMC Psychiatry 13:238, 2013Crossref, Medline, Google Scholar
21 : Weight maintenance following the STRIDE lifestyle intervention for individuals taking antipsychotic medications. Obesity 23:1995–2001, 2015Crossref, Medline, Google Scholar
22 : Body weight, image and self-esteem evaluation questionnaire: development and validation of a new scale. Schizophrenia Research 70:63–67, 2004Crossref, Medline, Google Scholar
23 : Measuring Health: A Guide to Rating Scales and Questionnaires. New York, Oxford University Press, 1996Google Scholar
24 : Reliability, validity, and application of the medical outcomes study 36-item Short-Form Health Survey (SF-36) in schizophrenic patients treated with olanzapine versus haloperidol. Medical Care 37:678–691, 1999Crossref, Medline, Google Scholar
25 : Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Services Research 39:1005–1026, 2004Crossref, Medline, Google Scholar
26 Stata Statistical Software: Release 13. College Station, Tex, StataCorp LP, 2013Google Scholar
27 : Improving lifestyle interventions for people with serious mental illnesses: qualitative results from the STRIDE study. Psychiatric Rehabilitation Journal (Epub ahead of print, July 2015)Google Scholar
28 : On stigma and its consequences: evidence from a longitudinal study of men with dual diagnoses of mental illness and substance abuse. Journal of Health and Social Behavior 38:177–190, 1997Crossref, Medline, Google Scholar
29 : Subjective experiences of stigma: a focus group study of schizophrenic patients, their relatives and mental health professionals. Social Science and Medicine 56:299–312, 2003Crossref, Medline, Google Scholar
30 : The effect of experimental presentation of thin media images on body satisfaction: a meta-analytic review. International Journal of Eating Disorders 31:1–16, 2002Crossref, Medline, Google Scholar
31 : Prevention of obesity and eating disorders: a consideration of shared risk factors. Health Education Research 21:770–782, 2006Crossref, Medline, Google Scholar
32 : Body image dissatisfaction among males across the lifespan: a review of past literature. Journal of Psychosomatic Research 56:675–685, 2004Crossref, Medline, Google Scholar
33 : Factors associated with body dissatisfaction and disordered eating in women in midlife. International Journal of Eating Disorders 43:527–536, 2010Crossref, Medline, Google Scholar
34 : Correlates of satisfaction with body function and body appearance in middle- and older-aged adults: the Activity Counseling Trial (ACT). Psychology and Health 15:239–254, 2000Crossref, Google Scholar



