Patients’ Preferences Related to Benefits, Risks, and Formulations of Schizophrenia Treatment
Abstract
Objective:
The objective of this study was to quantify patients’ preferences related to benefits and risks of antipsychotic treatments for schizophrenia and to assess the relative importance of treatment attributes and adherence.
Methods:
Treatment-related preferences among U.S. residents with a self-reported physician diagnosis of schizophrenia were assessed via a discrete-choice experiment. Patients chose between competing hypothetical scenarios characterized by improvements in positive symptoms, negative symptoms, and social functioning; incidence of weight gain, extrapyramidal symptoms (EPS), hyperprolactinemia, and hyperglycemia; and medication formulation. Preferences were estimated by using a random-parameters logit model, and the impact of adherence was estimated with conditional logit models.
Results:
The final sample consisted of 271 patients. Complete improvement in positive symptoms was the most preferred outcome (relative importance score of 10.0), followed by elimination of hyperglycemia (3.6, 95% confidence interval [CI]=2.6–4.6), improvement in negative symptoms (3.0, CI=1.6–4.3), reduced weight gain (2.6, CI=1.2–4.0), avoidance of hyperprolactinemia (1.7, CI=.9–2.6), improved social functioning (1.5, CI=.4–2.5), and avoidance of EPS (1.0, CI=.3–1.8). Patients judged a daily pill superior to monthly injections (p<.01) and monthly injections superior to injections every three months (p<.01) for adherent patients and monthly injections superior to a daily pill for nonadherent patients (p=.01).
Conclusions:
Persons who self-identified as having schizophrenia judged improvement in positive symptoms as the most important treatment benefit. Hyperglycemia was identified as the most important adverse event. Patients judged oral formulations to be better than monthly injections for adherent patients and monthly injections to be a better choice for nonadherent patients.
Schizophrenia is a major psychotic disorder, with symptoms including changes in perception, feeling, behavior, judgment, ideation, thought process, and motivation. Symptom manifestation is heterogeneous and variable over time (1). The lifetime prevalence of schizophrenia is approximately 1 in 100 (1), and the incidence of schizophrenia in the United States is 11.1 per 100,000 (2).
Treatment of schizophrenia with antipsychotics requires a balance between alleviation of symptoms and minimization of adverse events (3). Desired benefits include avoiding relapse or hospitalization, amelioration of positive and negative symptoms, and improvement in psychosocial and occupational functioning. Common risks include weight gain and metabolic disturbances (hyperglycemia, diabetes, and hyperlipidemia), extrapyramidal symptoms (EPS), and prolactin elevation. Treatment formulation is also important, especially considering the potential of long-acting injectables (LAIs) to simplify dosing regimens and improve outcomes among patients with poor adherence to medication (4).
Numerous choices of antipsychotic medications with various efficacy and side-effect profiles are available to the clinician. When deciding among treatments, physicians must consider both available evidence and the preferences of patients (5), which can be quantified by using stated-preference methods, such as discrete-choice experiments (DCEs), also known as conjoint analyses (6,7). Previous studies of antipsychotic preference have demonstrated that patients, physicians, and family members collectively place greater importance on productive activity (work or school) compared with positive symptoms or social functioning and place less importance on negative symptoms and side effects (8,9). Medication side effects are of greater concern among patients and their families than among clinicians (10–14).
Our study built on this research by identifying key attributes (benefits and risks) of antipsychotics and quantifying the trade-offs considered by patients when balancing attributes with formulation and adherence. Novel aspects of our work included use of a structured benefit-risk framework to identify a set of the most critical benefits and harms that physicians consider in antipsychotic treatment decisions, a quantitative approach to assess judgments for trade-offs between formulation and benefits, and a method for quantifying the impact of adherence on these trade-offs.
Methods
DCE Study
DCE studies quantify respondent preferences via a series of choice tasks requiring respondents to indicate which of several hypothetical treatment alternatives they prefer. Treatment alternatives are defined by systematically altering various treatment attributes, exposing respondents to hypothetical treatments that are reminiscent of but not representative of any existing treatment. DCEs are now common (7,15–17) and are increasingly applied in online surveys (6,7,16). Regression analysis of the association of respondents’ choices and the attributes of the treatment alternatives allows for an estimation of the relative importance of the attributes considered (6,18).
Study Sample
We targeted a sample of 300 patients, a sample size consistent with both current DCE guidelines (19,20) and current DCE practices in health (18). Respondents who were at least 18 years of age and who had a self-reported physician diagnosis of schizophrenia were identified via a prescreening survey and were recruited through Kantar Health’s online patient panel in May 2012. In total, 811 respondents received e-mail invitations to participate in the online survey. Respondents received “points” equivalent to 5–10 euros ($6–$13 U.S.) that could be redeemed for merchandise or services or donated to charity. The Office of Research Protection and Ethics at RTI International approved this study.
Survey Instrument
This survey was developed in conjunction with a similar instrument targeted to physicians (21,22). Applying an approach based in multicriteria decision analysis and the Benefit-Risk Action Team (BRAT) framework (23,24), we conducted written and telephone interviews with expert academic psychiatrists and assessed product inserts and publications to determine what attributes to include in the survey. Seven attributes were chosen, including improvements in three domains of symptoms (positive symptoms, negative symptoms, and social functioning) and incidence of four adverse events (weight gain, EPS, hyperprolactinemia [irregular periods or difficulty getting or maintaining erections], and hyperglycemia). To assess validity and reliability of our instrument, a draft version was tested in 12 open-ended cognitive interviews (25), after which minor changes were made to the wording to improve respondent comprehension.
Our survey used two types of choice tasks, one to assess treatment preferences and another to assess the impact of formulation and adherence. In the preference task, respondents randomly received one of six blocks of eight randomly ordered choice questions generated from a main-effects, D-optimal experimental design consisting of 48 paired treatments (26,27). Levels for positive and negative symptoms corresponded to the absent, mild, moderate, and severe levels in the Positive and Negative Syndrome Scale (PANSS), with descriptions based on symptom lists from the PANSS scoring convention. The choices consisted of a pair of treatments, each characterized by profiles of the seven attributes (Table 1); patients were asked to choose which treatment was better for “Pat,” a hypothetical patient with schizophrenia. [An example of a preference choice task question is available in an online supplement to this article.] Consistent with previous applications of DCEs among patients with schizophrenia (10), respondents’ preferences were elicited by making a judgment about which choice is better for a third party.
| Attribute | Level | |||
|---|---|---|---|---|
| Symptom domain | None | Mild | Moderate | Severe |
| Positive symptoms | No unusual beliefs, doesn’t hear voices, trusts people | Thinks someone is following them, sometimes hears voices, has difficulty trusting people | Thinks people are stealing from them, often hears voices, doesn’t trust people | Thinks government hid listening device in them, hears voices all the time, stays home with a weapon |
| Negative symptoms | Speaks normally, has emotions | Speaks briefly, doesn’t laugh | Speaks rarely and slowly, rarely shows emotion | Speaks only when asked, shows no emotion |
| Social functioninga | Less interest in social activities (noticed by family or close friends) | Little interest in social activities (needs to be encouraged) | No interest in social activities | |
| Adverse event | No | Yes | ||
| Weight gain | No weight gain over the past year | 15-pound weight gain over the past year or 30-pound weight gain over the past year | ||
| Extrapyramidal symptoms | No muscle problems | Muscle stiffness or shaking | ||
| Hyperprolactinemia | No problems with erections (men); normal periods (women) | Problems getting and maintaining erections (men); irregular periods (women) | ||
| Hyperglycemia | Normal blood sugar | Has high blood sugar that can lead to diabetes | ||
TABLE 1. Attributes associated with antipsychotic treatment for schizophrenia contained in survey preference choice tasks
In the adherence task, respondents randomly received one of nine blocks of four randomly ordered choice tasks considering the trade-off between formulation (daily pill, monthly injection, or injection every three months) and risk of experiencing mild and severe positive symptoms (Table 2). Positive symptoms were assessed by using three distributions of mild and severe symptoms (50%/50%, 40%/60%, and 20%/80%). In these tasks, the same treatments were compared for both an adherent patient (“Pat”) and a patient who misses his oral antipsychotic medications (“Jaime”). [An example of a formulation and adherence choice task question is available in the online supplement.]
| Attribute | Level |
|---|---|
| How Pat takes the medicine (mode of administration) | Injection once every 3 months; injection once a month; pill once a day |
| Having unusual beliefs, hearing voices, and not trusting people (positive symptoms) | Three combinations of mild and severe symptoms: mild: 5 out of 10 (50%), severe: 5 out of 10 (50%); mild: 4 out of 10 (40%), severe: 6 out of 10 (60%); or mild: 2 out of 10 (20%), severe: 8 out of 10 (80%) |
TABLE 2. Attributes and levels associated with antipsychotic treatment for schizophrenia contained in survey choice tasks
Statistical Analysis
Responses to the preference choice tasks were analyzed by using a random-parameters logit model, in which a regression examined the association of respondents’ choices and attribute levels in each scenario, allowing estimation of the relative importance of each attribute level (6,25,28). For the adherence choice tasks, conditional logit models were used to estimate the relative importance weights for formulation and chance of improvement in positive symptoms, given information regarding patient adherence history. All analyses were conducted by using NLOGIT 4.0.
Results
Sample Characteristics
Of the 811 patients invited to participate, 684 (84%) responded and 329 (41%) were eligible and provided informed consent. Of eligible respondents who consented, 301 (91%) answered at least one choice question.
Of these 301 respondents, 30 chose the same response (medicine A or B) for all eight preference choice questions. Given the random assignment of attribute profiles into column A or B, this pattern should occur for only 2.4 respondents, suggesting that these 30 respondents did not focus on the survey (29). Data from these respondents were excluded because of validity concerns, leaving 271 respondents in the final sample.
Table 3 summarizes the demographic and clinical characteristics of the final sample: 73% were white, 60% were male, 33% were married, 60% were diagnosed as having schizophrenia between 15 and 25 years of age, and 90% took prescription medication for schizophrenia; the mean±SD age was 38.4±11.9 years. Compared with participants in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study (30), our sample reflected greater rates of employment, married status, white race, female gender, and use of prescription medication, and more years of education. Significance tests showed that greater educational attainment (high school or less versus some college or more) was not associated with statistically significant differences in preferences.
| Survey (N=271) | CATIE (N=1,460) | |||
|---|---|---|---|---|
| Characteristic | N | % | N | % |
| Male | 163 | 60 | 1,080 | 74 |
| Married | 90 | 33 | 167 | 11 |
| White | 199 | 73 | 874 | 60 |
| Employedb | 112 | 41 | 218 | 15 |
| Age (M±SD) | 38.4±11.9 | 40.6±11.1 | ||
| Education (M±SD years)c | 13.9 | 12.1±2.3 | ||
| Diagnosed as having schizophrenia between 15 and 25 years of age | 162 | 60 | ||
| Age at first treatment for any behavioral or emotional problem | — | 24.0±8.9 | ||
| Taking prescription medication | 245 | 90 | 1,046 | 72 |
TABLE 3. Characteristics of 271 survey respondents and 1,460 participants in the CATIE studya
Mean Relative Importance Weights for Outcomes
Statistical analysis of the preference choice tasks indicated that survey participants considered improvement in positive symptoms the most important outcome (Figure 1). That is, a treatment associated with an improvement in level of positive symptoms from severe to none and that did nothing else would provide more perceived benefit than a treatment that improved any other attribute over the range of levels studied. This change was assigned an importance value of 10.0.
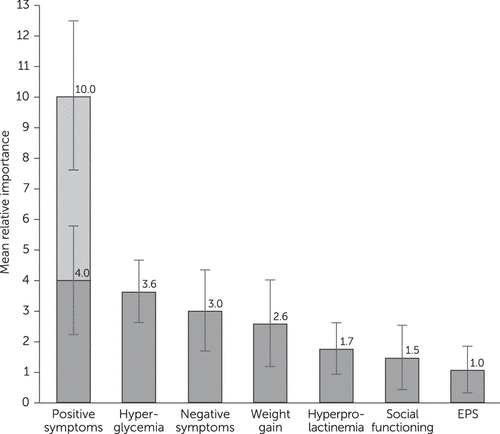
FIGURE 1. Relative importance weights for seven attributes associated with antipsychotic treatment for schizophreniaa
a The attributes were improvements in positive symptoms, negative symptoms, and social functioning and incidence of hyperglycemia, weight gain, hyperprolactinemia, and extrapyramidal symptoms (EPS). The vertical lines represent the 95% confidence intervals around the mean estimates. The lower part of the positive symptoms bar shows the preference associated with a change from severe to moderate positive symptoms. The upper part of the bar shows the preference associated with a change from moderate to no positive symptoms.
The second most important feature was eliminating hyperglycemia, which had a mean relative importance of 3.6 (95% confidence interval [CI]=2.6–4.6), indicating that avoiding hyperglycemia was approximately one-third as important as complete improvement in positive symptoms (p<.05). The relative importance of the other attributes, listed in order of decreasing importance, was improvement in negative symptoms from severe to none (3.0, CI=1.6–4.3), 30-pound weight gain (2.6, CI=1.2–4.0), hyperprolactinemia (1.7, CI=.9–2.6), improvement in social functioning from severe to mild (1.5, CI=.4–2.5), and EPS (1.0, CI=.3–1.8). The adverse event with the greatest relative importance was hyperglycemia, which was considered more than 3.5 times as important as the adverse event with least relative importance, avoiding EPS (p<.05).
The model also yielded insights on the changes within an attribute that were most important (data not shown). For weight gain, an increase of 15 to 30 pounds was three times more important than an increase of 0 to 15 pounds. For negative symptoms, the only statistically significant difference in importance was a change from mild to no symptoms. In contrast, for positive symptoms, the changes between levels were of similar importance.
Weights for Formulation and Adherence
The adherence choice tasks inquired separately about two levels of nonadherence: missing one to two doses per week and missing three to four doses per week (roughly missing 20% and 50% of doses, respectively). Because there was no difference between the results for these two levels of nonadherence, the results for both responses were pooled and referred to as nonadherent.
Preference weights showed different patterns for an adherent and a nonadherent patient (Figure 2). For an adherent patient, respondents preferred a daily pill to an equally effective monthly injection (p<.01) and preferred monthly injection to three-month injection (p<.01). For a nonadherent patient, respondents preferred monthly injection to a pill (p=.01). The preference difference between monthly and 3-month injections for a nonadherent patient was not statistically significant.
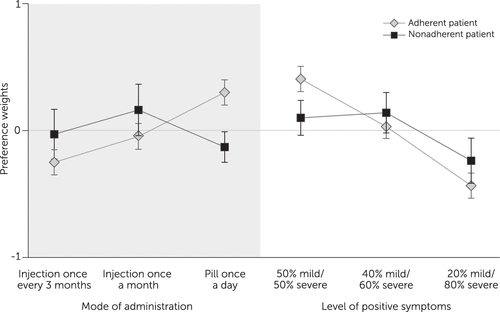
FIGURE 2. Relative importance weights for switching between formulations of antipsychotic treatment for schizophrenia and between rates of levels of positive symptoms among adherent and nonadherent patientsa
a Positive changes in importance weights indicate increases in preference. Vertical lines show 95% confidence intervals around the mean preference estimate. Trade-offs between formulation and efficacy were estimated by comparing the change in preference (the vertical distance) between two formulations (left) with the change in preference between two levels of efficacy (right). For example, for nonadherent patients, the preference change between oral formulation and monthly injection is equivalent to almost all of the preference change between 20% efficacy and 50% efficacy, resulting in the formulation change being preferentially equivalent to a change in efficacy of 26%.
Finally, the clinical value of an injectable formulation for nonadherent patients can be inferred by comparing the change in preference for switching between an oral and injectable with the change in preference for achieving a given reduction in positive symptoms (Figure 2). Participants indicated that for a nonadherent patient, an increase of up to 26% (CI=3%−49%) in the chance of severe positive symptoms was an acceptable trade-off for switching from an oral formulation to a one-month injectable. For adherent patients, because the oral formulation was preferred over injectables, participants indicated that to be an acceptable trade-off, switching from an oral formulation to a monthly injectable would require a reduction of at least 15% (CI=7%−23%) in the chance of severe positive symptoms.
Discussion
Understanding the importance that patients place on the benefits and risks of antipsychotics and how formulation affects those trade-offs provides insight into past decisions and useful information for future regulatory and treatment decisions. This study built on prior work in this area in three key ways. First, by using a structured benefit-risk approach with input from key opinion leaders and a literature review, we identified a key set of benefits and risks that physicians consider when making decisions about antipsychotic treatment. Second, by incorporating formulation into the choice questions, we obtained quantitative estimates of respondents’ willingness to accept trade-offs between formulation and degree of benefit. Third, by providing information on a hypothetical patient’s adherence, we assessed how perceptions of adherence affected these trade-offs.
Patients regarded complete removal of positive symptoms as more important than any other symptom or adverse event assessed. Notably, an improvement in positive symptoms from severe to moderate levels was as important as or more important than improvement in any other adverse event included (Figure 1). This finding suggests that the main driver in antipsychotic treatment decisions is stopping severe positive symptoms. Once a patient with severe positive symptoms shows some improvement, the trade-off between improved efficacy and adverse events becomes more important. Although the rationale behind these measurements was not examined, one possible explanation is that patients understand the dangers associated with severe positive symptoms more than those for negative symptoms, and they may view adverse events as amenable to control by adjustments in dose, choice of antipsychotic, or both.
Other findings related to the value respondents placed on switching from an oral antipsychotic to a LAI. As might be expected, this value depended on the hypothetical patient’s adherence behavior. For adherent patients, oral formulations were judged superior to injectables. For nonadherent patients, respondents showed a statistically significant judgment in favor of a one-month LAI over an oral form. The importance of switching to a monthly LAI was similar to the importance of a 26% change in chance of reducing the level of severe positive symptoms. In other words, given the choice between a highly effective oral drug and a somewhat less effective LAI, respondents would choose the LAI for nonadherent patients. If confirmed, such results may be useful for regulatory decision making and clinical practice.
A surprising result is related to patient perceptions about improvements in negative symptoms. An improvement in negative symptoms was considered important only for the elimination of mild symptoms, not for improvements of severe or moderate symptoms to moderate or mild levels. Although patients noted distinctions among the survey descriptions of severe, moderate, and mild negative symptoms (Table 1) during pretesting, they did not value changes in these levels of severity while taking the survey. This finding may be a consequence of differences between viewpoints of patients and physicians and patients’ limited understanding of the effect of negative symptoms. The absence of approved treatments for negative symptoms (and social functioning) could diminish understanding of these symptoms and the value placed on them, given that there is not much real-world expectation for improvement.
The findings of this study were similar to those of other published studies in which respondents valued improvements in social functioning and positive symptoms more than improvement in negative symptoms and avoidance of side effects (8,9). A related study in which the authors assessed psychiatrist judgments about medications’ benefits and risks showed that the psychiatrists and patients had several results in common (21,22). Like patients, psychiatrists showed that positive symptoms were their dominant concern. Psychiatrists showed little difference in their opinions about the importance of various formulations for an adherent patient, but as adherence decreased, psychiatrists preferred both one-month and three-month injectables over oral formulations (p<.01). Like patients, psychiatrists would accept up to a 20% to 25% reduction in efficacy in order for a highly nonadherent patient (missed 50% of doses) to switch from a monthly injectable to an oral formulation.
An important consideration is the degree to which the judgments of a panel of patients can be used in the context of individual patient treatment. Mean results from a panel may not be informative about individual preferences. However, panel results can be considered in a manner similar to the consideration of clinical data in treatment guidelines. Both provide evidence about patient populations and enable physicians to better compare the small samples they treat with the larger populations described in the guidelines. Treatment decisions potentially can then be based on both clinical study data and preference data. Mean preference results may be particularly valuable when treating a patient who chooses not to indicate personal preferences or is incapable of doing so.
There were several noteworthy limitations of this study. First, we designed the survey to help respondents interpret the attributes consistently and as intended. However, evaluating choice tasks could be cognitively difficult for patients with schizophrenia, although recent work suggests that is not the case (10). A training section of the survey provided attribute definitions and practice questions. Second, as in all DCE studies, experimental control over the decision stimuli required respondents to evaluate hypothetical choice alternatives. Thus there was potential for hypothetical bias. The study minimized hypothetical bias by using patient-friendly descriptions of treatment attributes that reflected clinically realistic outcomes.
A third limitation was that constraints on cognition limited the number of endpoints that could be considered simultaneously by survey respondents. We used a structured approach for endpoint selection to mitigate this limitation.
Fourth, unlike positive and negative symptoms, social functioning did not include a level for no symptoms or complete cure. This level was excluded because, when pretesting the physician survey on which this patient survey was based, physicians were unable to accept a scenario in which negative symptoms were unaccompanied by limitations on social functioning. We posited that patients would have a similar concern. If the full range of social functioning was included, social functioning might have shown greater importance compared with negative symptoms. Additionally, using alternative definitions of social functioning that reference jobs, independent housing, or time with friends or family may have led participants to attach greater importance to this attribute.
Fifth, this study surveyed a convenience sample of patients from the United States with access to the Internet. This design limits the confidence with which these results can be generalized. As suggested by Table 3, this sample represents a more educated population than is typical for patients with schizophrenia. Future research using a randomized patient sample to verify our findings would be valuable. Readers should also understand that these relationships may change over time as understanding of schizophrenia and its treatment evolves.
Sixth, this study was not designed, nor powered, to address the question of whether judgments would change depending on experience with a particular adverse event, such as EPS, or with adherence challenges. Any potential differences were averaged out. However, a post hoc test on results for the 198 patients who answered a question about self-assessed adherence showed no difference between the groups who did or did not adhere to medication. With a larger sample size, a study could show meaningful differences in judgment on the basis of personal or family medical history and could potentially provide better guidance for individual patient treatment.
Seventh, the survey assessed judgment, which is distinct from personal preference or choice. Schizophrenia patients are very sensitive about revealing personal information, and prior DCE work showed that such patients answer hypothetical questions about others (judgments) more easily than about themselves (preferences) (10). Formulating the questions as judgments also avoided confusion with patients’ experiences and expectations about how treatments could affect their own emotional responses, encouraged objectivity, and reduced potential yea-saying bias compared with a question about what patients would choose for themselves (10,31–34). Use of the results as indicators for personal treatment decisions assumes that the respondents’ judgments are a valid representation of personal preference.
Finally, although DCE surveys could be conducted through in-person interviews, many researchers believe that computerized administration assures respondents of anonymity and reduces yea-saying and interviewer bias. Results from online DCE studies are generally not statistically significantly different from those elicited through face-to-face interviews (35,36), and several DCE studies using online patient panels have been published (6,7,16,37).
Conclusions
Balancing the benefits and risks of treatments is the core of treatment decisions by health authorities, physicians, and patients. This study demonstrated that patients evaluated treatments primarily on the basis of improvement in positive symptoms and that hyperglycemia was judged the most important adverse event. Patients judged oral formulations to be better for adherent patients but judged a monthly injection to be better for nonadherent patients. The results are consistent with our prior DCE study with psychiatrists. Studies of this type can help in understanding the importance people place on the benefits and risks of antipsychotics and how formulation affects those trade-offs, providing insight for both regulatory approval and shared decision making between patients and physicians.
1 : Practice guideline for the treatment of patients with schizophrenia, 2nd ed. American Journal of Psychiatry 161(Feb suppl):1–56, 2004Link, Google Scholar
2 : Prevalence and incidence studies of schizophrenic disorders: a systematic review of the literature. Canadian Journal of Psychiatry 47:833–843, 2002Crossref, Medline, Google Scholar
3 : Oral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-making. Expert Opinion on Pharmacotherapy 10:1917–1928, 2009Crossref, Medline, Google Scholar
4 : New second-generation long-acting injectable antipsychotics for the treatment of schizophrenia. Expert Review of Neurotherapeutics 13:767–783, 2013Crossref, Medline, Google Scholar
5 : Evidence-based medicine: it’s not just about the evidence. International Journal of Clinical Practice 65:634–635, 2011Crossref, Medline, Google Scholar
6 : A discrete-choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthritis and Cartilage 21:289–297, 2013Crossref, Medline, Google Scholar
7 : Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer 77:224–231, 2012Crossref, Medline, Google Scholar
8 : Preferences for schizophrenia treatment outcomes among public policy makers, consumers, families, and providers. Psychiatric Services 54:1124–1128, 2003Link, Google Scholar
9 : Assessing preferences for schizophrenia outcomes: comprehension and decision strategies in three assessment methods. Mental Health Services Research 5:121–135, 2003Crossref, Medline, Google Scholar
10 : Can patients diagnosed with schizophrenia complete choice-based conjoint analysis tasks? Patient 4:267–275, 2011Crossref, Medline, Google Scholar
11 : A test of concordance between patient and psychiatrist valuations of multiple treatment goals for schizophrenia. Health Expectations 16:164–176, 2013Crossref, Medline, Google Scholar
12 : Valuation and attainment of treatment goals in schizophrenia: perspectives of patients, relatives, physicians, and payers. Journal of Psychiatric Practice 18:321–328, 2012Crossref, Medline, Google Scholar
13 : Preference weights for cost-outcome analyses of schizophrenia treatments: comparison of four stakeholder groups. Schizophrenia Bulletin 29:257–266, 2003Crossref, Medline, Google Scholar
14 : Measuring preferences for schizophrenia outcomes with the time tradeoff method. Journal of Behavioral Health Services and Research 32:14–26, 2005Crossref, Medline, Google Scholar
15 : Magnetic resonance imaging for the investigation of knee injuries: an investigation of preferences. Health Economics 7:595–603, 1998Crossref, Medline, Google Scholar
16 : Factors that affect adherence to bipolar disorder treatments: a stated-preference approach. Medical Care 45:545–552, 2007Crossref, Medline, Google Scholar
17 : Discrete choice experiments in health economics: a review of the literature. PharmacoEconomics 32:883–902, 2014Crossref, Medline, Google Scholar
18 : Conjoint analysis applications in health—how are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. Patient 3:249–256, 2010Crossref, Medline, Google Scholar
19 Johnson FR, Yang J-C, Mohamed AF: In defense of imperfect experimental designs; statistical efficiency and measurement error in choice-format conjoint analysis. Presented at the Proceedings of the Sawtooth Software Conference, Orlando, Fla, March 21–23, 2012Google Scholar
20 : Introduction to stated preference models and methods; in Stated Choice Methods: Analysis and Application. Edited by Louviere JJ, Hensher DA, Swait JD. Cambridge, United Kingdom, Cambridge University Press, 2000Crossref, Google Scholar
21 Markowitz M, Levitan B, Mohamed AF, et al: Psychiatrists’ preferences for benefit and risk outcomes and formulation in schizophrenia treatments: a conjoint analysis study. Presented at the Institute on Psychiatric Services, New York, Oct 4–7, 2012Google Scholar
22 : Psychiatrists’ judgments about antipsychotic benefit and risk outcomes and formulation in schizophrenia treatment. Psychiatric Services 65:1133–1139, 2014Link, Google Scholar
23 : Multi-Criteria Analysis: a Manual. London, United Kingdom, Department for Communities and Local Government, 2009Google Scholar
24 : Application of the BRAT framework to case studies: observations and insights. Clinical Pharmacology and Therapeutics 89:217–224, 2011Crossref, Medline, Google Scholar
25 : Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health 14:403–413, 2011Crossref, Medline, Google Scholar
26 : Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value in Health 16:3–13, 2013Crossref, Medline, Google Scholar
27 : Marketing Research Methods in SAS: Experimental Design, Choice, Conjoint, and Graphical Techniques. Cary, NC, SAS Institute, 2010Google Scholar
28 : Patient benefit-risk preferences for targeted agents in the treatment of renal cell carcinoma. PharmacoEconomics 29:977–988, 2011Crossref, Medline, Google Scholar
29 : How do physicians weigh benefits and risks associated with treatments in patients with osteoarthritis in the United Kingdom? Journal of Rheumatology 39:1056–1063, 2012Crossref, Medline, Google Scholar
30 : Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209–1223, 2005Crossref, Medline, Google Scholar
31 : Estimating importance weights for the IWQOL-Lite using conjoint analysis. Quality of Life Research 19:701–709, 2010Crossref, Medline, Google Scholar
32 : A Bayesian truth serum for subjective data. Science 306:462–466, 2004Crossref, Medline, Google Scholar
33 : The effects of response mode and importance on decision making strategies: judgment versus choice. Organizational Behavior and Human Performance 41:1–19, 1988Crossref, Google Scholar
34 : Social distance, framing, and judgment: a construal level perspective. Human Communication Research 33:489–514, 2007Crossref, Google Scholar
35 : Testing for the survey mode effect on contingent valuation data quality: a case study of Web-based versus in-person interviews. Ecological Economics 62:388–398, 2007Crossref, Google Scholar
36 : Use of the Internet for willingness-to-pay survey: a comparison of face-to-face and Web-based interviews. Resource and Energy Economics 33:119–129, 2011Crossref, Google Scholar
37 : Evaluation of the relative importance of chemotherapeutic and antiemetic efficacy in various oncologic settings. Supportive Care in Cancer 17:405–411, 2009Crossref, Medline, Google Scholar



