Animal-Assisted Therapy With Chronic Psychiatric Inpatients: Equine-Assisted Psychotherapy and Aggressive Behavior
Abstract
Objective:
Animal-assisted therapy (AAT), most frequently used with dogs, is being used increasingly as an adjunctive alternative treatment for psychiatric patients. AAT with larger animals, such as horses, may have unique benefits. In this randomized controlled study, equine and canine forms of AAT were compared with standard treatments for hospitalized psychiatric patients to determine AAT effects on violent behavior and related measures.
Methods:
The study included 90 patients with recent in-hospital violent behavior or highly regressed behavior. Hospitalization at the 500-bed state psychiatric hospital was two months or longer (mean 5.4 years). Participants were randomly selected to receive ten weekly group therapy sessions of standardized equine-assisted psychotherapy (EAP), canine-assisted psychotherapy (CAP), enhanced social skills psychotherapy, or regular hospital care. Participants’ mean age was 44, 37% were female, 76% had diagnoses of schizophrenia or schizoaffective disorder, and 56% had been committed involuntarily for civil or forensic reasons. Violence-related incident reports filed by staff in the three months after study intake were compared with reports two months preintake.
Results:
Interventions were well tolerated. Analyses revealed an intervention group effect (F=3.00, df=3 and 86, p=.035); post hoc tests showed specific benefits of EAP (p<.05). Similar AAT effects were found for the incidence of 1:1 clinical observation (F=2.70, df=3 and 86, p=.051); post hoc tests suggested benefits of CAP (p=.058) as well as EAP (p=.082). Covariance analyses indicated that staff can predict which patients are likely to benefit from EAP (p=.01).
Conclusions:
AAT, and perhaps EAP uniquely, may be an effective therapeutic modality for long-term psychiatric patients at risk of violence.
State psychiatric hospitals continue to treat many patients for whom traditional interventions have not led to successful community living, aggression being among the most challenging behavioral barriers. Animal-assisted therapy (AAT), a novel approach to treating major psychiatric disorders, has been reported to reduce anxiety and depression and enhance socialization of psychiatric patients (1–3). There are, however, few systematic studies of these treatments (4).
Dogs have become increasingly common therapy assistants; larger animals, such as horses, however, may be especially therapeutic for aggressive (and regressed) behavior. This may result from interacting with more imposing animals, with feasibility enhanced by the ready adaptability of horses to group exercises. Equine-assisted therapies can be costly and labor intensive, underscoring the need for studies of efficacy (5,6).
AAT has been used for more than a decade at the 500-bed state psychiatric hospital that was the site for this study, and equine-assisted psychotherapy (EAP) was piloted there in 2007. To assess equine (and canine) AAT feasibility and efficacy, we undertook a randomized controlled study that included EAP, canine-assisted psychotherapy (CAP), and both active and standard control conditions. Standard therapeutic approaches for each modality were used, with minor adaptations. This report focuses on AAT effects on violence and associated symptoms during the three months after initiation of the interventions.
Methods
The project was approved by the New Jersey Division of Human Services and the University of Medicine and Dentistry Institutional Review Board.
Participants
Inpatients at Greystone Park Psychiatric Hospital (GPPH), ≥18 and <65 years, were identified from hospital records and by treatment teams asked to identify patients with aggressive or regressed behavior, which was defined as three or more violent incidents in the preceding 12 months (or two incidents plus clinically perceived active risk) or persistent social isolation and difficulty engaging in discharge-related programs (most in a database maintained by social services of patients discharge-ready for more than 12 months). A large majority of GPPH patients met one or both criteria; the intent was to be inclusive. Exclusion criteria, determined on medical director review, included impaired ambulation, cognitive impairments, or other medical factors that might be exacerbated or result in harm during animal contact. Patients were not referred if their behavior was considered to pose a substantive risk to animals or peers.
Design and Protocol
Introductory presentations to potential participants on each long-term unit were followed by written informed consent after complete description of the study. Each participant was randomly assigned to one of four groups: EAP, CAP, environmentally enhanced social skills group psychotherapy (SSP) (active control), or regular hospital care (standard control). Active interventions provided for ten 40- to 60-minute weekly group sessions, with groups of up to ten members that were homogeneous on recruitment subtype (aggressive or regressed). EAP, CAP, and SSP were conducted in parallel at 9 a.m., 10:45 a.m., and 1 p.m. Participants in the standard control group received no additional interventions beyond regular hospital treatment. Groups were conducted at dedicated sites on hospital grounds, including suitably configured outdoor areas (EAP) or cottages (CAP and SSP). EAP and CAP session staffing included certified pet therapists, hospital rehabilitation staff, and hospital staff to address emergent clinical issues. Aside from the additional weekly AAT activity, participants’ hospital care was unchanged. The two months preceding enrollment were used as baseline for hospital-derived data; clinical instruments were completed by staff members, who were blind to group assignment, at intake. Outcomes reflected the three months after study initiation.
Animal-Assisted Interventions
EAP.
EAP followed the model of the Equine Assisted Growth and Learning Association (EAGALA) (7), a nonprofit association for professionals using equine therapy to address mental health and human development needs. Two EAGALA-certified equine therapists (having extensive experience with therapy for people with physical disabilities) worked with two or three therapy horses tested and credentialed as suitable for direct patient contact in clinical environments by Delta Society/Pet Partners. Sessions were conducted in an area adjacent to the hospital with a specially designed corral. Interventions included scripted, increasingly complex ground exercises involving group interactions among patients, horses, and the equine therapists. Patients’ interactions with horses included no riding. Session protocols and treatment duration followed EAGALA and Delta Society principles extrapolated from the equine therapists’ experiences and those of other treatment centers, and they accommodated animal availability, hospital schedules, and animal fatigue.
A typical midprotocol session involved reviewing safety, greeting the horses, discussing preceding sessions, and working on an activity, such as designing a course using cones through which horses would be led by two or three participants with designated roles. Discussion of the horses’ and the patients’ own responses preceded closing interactions with the horses.
CAP.
Three certified therapist-and-dog teams from St. Hubert’s Animal Welfare Center in Madison, New Jersey, were selected. Sessions were intended to provide comparability to EAP in their novelty and environmental change from the main hospital. Common CAP models provide graduated unstructured interactions between dogs and patients. To maximize comparability with EAP, the CAP model selected was a more structured group therapy, with animal greeting, discussion, and exercises, such as grooming, leading, and directing the dogs.
SSP
The active control group involved social skills exercises identical to those in the hospital’s general program, but, as with CAP, they were conducted in an appropriately configured cottage. The setting was intended to control therapeutic factors unrelated to animal interactions, including leaving the hospital building, proceeding to a novel setting, and having added staff attention.
Regular Hospital Care
Participants in the standard control group remained in the general hospital, receiving no additional intervention except recruitment procedures and very brief three-month follow-up assessments.
Assessments and Outcomes
Data from hospital records contrasted the two months preceding intake with the three months postintake. Intake interview measures were obtained from staff with an ongoing clinical relationship with the patient but blind to AAT assignment and compared with measures three months postintake. Staff training sessions on the measures suggested consensus; however, obtaining formal interrater reliability ratings was not feasible. Potential covariates among the clinical and demographic characteristics included age, sex, chart-derived psychiatric and medical diagnoses, hospitalization duration, legal commitment status, and number of intervention sessions attended. Although formal diagnostic assessment using standardized interviews would have been desirable, such assessments were not feasible and not considered essential to the study’s behavioral goals and rationale. Staff members interviewed at follow-up were not blind to patient project involvement; however, many (47%, N=41 of 88 responders) reported limited awareness of AAT assignments, and none had direct involvement in AAT interventions.
Aggression-related outcome measures.
The primary outcome was frequency of aggressive behavior identified by hospital incident reports, filed independently by nursing staff as a hospital mandate. Incidents were categorized as violent or not by an investigator blind to group assignment and who used hospital staff–coded categories and the descriptive text in the reports. Nonviolent incidents were quantified as a nonspecific comparison variable. Other outcomes included the frequency at which patients required 1:1 clinical observation or seclusion or restraint. Secondary measures assessed by staff included verbal and physical aggression on the Overt Aggression Scale (OAS-M [8]; highest score on items 1–3).
Other clinical and functional measures.
Secondary outcomes and potential mediators of aggression effects were mostly staff-rated measures obtained at intake and three months; these included the Brief Psychiatric Rating Scale (BPRS [9]); the Life Skills Profile (LSP-20; high scores were reverse coded for uniformity to indicate lower function [10]); the Greystone Intrusiveness Measure (GIM [11]), a Likert-type measure of the patient’s propensity to violate others’ personal “space”; staff expectations that AAT would benefit the patient; the Pet Attitude Scale–Modified (12), completed by the patient; and visual analog scales (13), scored by both staff and patients, for quantifying current anxiety, depression, anger, and isolation.
Data Analyses
Statistical analyses used SPSS (12.0); all tests were two-tailed. To examine intervention effects, these analyses excluded participants assigned to EAP, CAP, or SSP who did not attend any sessions. As a conservative analytic approach, data for all other participants (meaning those with any exposure to the interventions) were included. Intervention group differences in baseline clinical and demographic characteristics were assessed. Outcomes among the four intervention groups were assessed primarily with analysis of variance (ANOVA) in generalized linear models (Tukey post hoc tests). Covariance analyses assessed effects in the violent incidents models.
Results
Participants and Session Attendance
Of 105 inpatients (from 20 clinical units) signing consent, one was deceased prior to project initiation, 12 did not attend any intervention sessions, and two had insufficient baseline data (admitted less than two months before study intake). Characteristics of the remaining 90 participants are presented in Table 1. Participants’ mean age was 44, 37% were women, 61% were non-Latino Caucasian, and 76% had chart diagnoses of schizophrenia or schizoaffective disorder. Mean hospitalization length was 5.4 years, 56% had civil commitment status or had been committed on the basis of a finding of not guilty by reason of insanity, and 63% were identified for the study primarily for aggressive behavior. There were no significant baseline differences among the four intervention groups (ANOVA or chi square) in age, sex, racial-ethnic background, diagnosis, days from hospital admission to study intake, recruitment for aggressive versus regressed behavior, aggressive incidents in the two months preceding intake, BPRS score, and attitudes toward pets and other animals. Baseline differences were found for the LSP-20 (F=2.83, df=3 and 85, p<.05) and OAS-M items assessing assault against objects (F=2.87, df=3 and 85, p<.05) and global overt irritability (F=3.57, df=3 and 83, p<.02). Post hoc tests found higher baseline OAS-M aggression and life skills dysfunction for EAP- versus CAP-assigned participants (p<.05). Staff expectations of AAT benefit, non–aggression-related incident reports, 1:1 staff observation, seclusion and restraint, visual analog scales, and intrusiveness (GIM) also showed no baseline differences among groups (data not shown).
| Canine assisted (N=25)a | Equine assisted (N=24)a | Enhanced social skills (N=23)a | Regular hospital care (N=18) | Total (N=90) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | N | % | N | % |
| Age | 45.0±10.8 | 44.3±13.8 | 43.2±10.3 | 45.2±13.4 | 44.4±11.9 | |||||
| Female | 11 | 44 | 6 | 25 | 10 | 43 | 6 | 33 | 33 | 37 |
| Background | ||||||||||
| Caucasian | 17 | 68 | 15 | 63 | 13 | 57 | 10 | 56 | 55 | 61 |
| African American | 4 | 16 | 5 | 21 | 6 | 26 | 5 | 28 | 20 | 22 |
| Latino | 4 | 16 | 4 | 17 | 4 | 17 | 3 | 17 | 15 | 17 |
| Chart diagnosis | ||||||||||
| Schizophrenia | 8 | 32 | 7 | 29 | 8 | 35 | 7 | 39 | 30 | 33 |
| Schizoaffective disorder | 14 | 56 | 9 | 38 | 10 | 43 | 5 | 28 | 38 | 42 |
| Affective or other disorder | 3 | 12 | 8 | 33 | 5 | 22 | 6 | 33 | 22 | 24 |
| Hospitalization from admission to intake (days) | 1,568±2,150 | 2,173±2,361 | 2,053±1,649 | 2,105±2,289 | 1,961±2,103 | |||||
| Involuntary or forensic status | 13 | 52 | 13 | 54 | 15 | 65 | 9 | 50 | 50 | 56 |
| Entered study on basis of violence history | 15 | 60 | 16 | 67 | 17 | 74 | 9 | 50 | 57 | 63 |
| Aggressive events 2 months before intake | .66±1.03 | 1.33±2.04 | .98±1.11 | .69±.96 | .93±1.39 | |||||
| Brief Psychiatric Rating Scale score (BPRS)b | 45.6±16.0 | 50.5±18.5 | 51.3±17.4 | 42.7±11.8 | 47.8±16.5 | |||||
| Life Skills Profile–20 scorec | 40.0±7.9 | 47.0±8.2 | 41.7±9.4 | 41.2±1.8 | 42.6±9.3 | |||||
| Pet Attitude Scale scored | 100.9±11.2 | 92.2±18.2 | 94.5±20.5 | 99.3±13.3 | 96.6±16.5 | |||||
Table 1 Baseline characteristics of 90 patients randomly assigned to animal-assisted therapy, social skills therapy, or regular hospital care
AAT interventions were well tolerated with no adverse effects requiring medical or psychiatric attention. Sessions ran from mid-March to June 2010; attendance appeared sensitive to weather conditions. The median number of sessions attended was seven each for EAP and CAP and five for SSP; there were no significant differences across groups. Eighty-three percent of EAP (N=20), 80% of CAP (N=20), and 78% of SSP (N=18) participants attended at least three sessions. Of the 90 patients, four were discharged before conclusion of the interventions (one from each group), and three were transferred to a more restrictive forensic hospital (one from CAP and two from SSP).
AAT and Aggressive Behavior
As shown in Table 2, the four groups differed when the mean number of violence-related incident reports for the two months preceding study intake was compared with that for the three postintake months (p=.035). Violent incident reports (per patient per month) suggested decreases for EAP patients but increases for other groups (Figure 1 and Table 2). Post hoc tests revealed significant differences contrasting EAP and SSP. There was no evidence that CAP reduced violence-related incidents (including use of less stringent least-significant-difference post hoc tests, in which EAP showed reduced incidents compared with incidents reported for each of the other groups, including CAP [data not shown]).
| Canine assisted (N=25) | Equine assisted (N=24) | Enhanced social skills (N=23) | Regular hospital care (N=18) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure and time point | M | SD | M | SD | M | SD | M | SD | F | df | p |
| Monthly violent incidents | 3.00 | 3, 86 | .035 | ||||||||
| 2 months preintake | .66 | 1.03 | 1.33 | 2.04 | .98 | 1.11 | .69 | .96 | |||
| 3 months postintake | .99 | 1.40 | .77 | .87 | 1.67 | 2.54 | 1.00 | 1.73 | |||
| Monthly nonviolent incidents | .29 | 3, 86 | >.1 | ||||||||
| 2 months preintake | .24 | .29 | .33 | .52 | .30 | .88 | .47 | .58 | |||
| 3 months postintake | .30 | .58 | .33 | .51 | .36 | .67 | .43 | .55 | |||
| Monthly 1:1 observation | 2.70 | 3, 86 | .051 | ||||||||
| 2 months preintake | .22 | .52 | .19 | .38 | .26 | .50 | .22 | .57 | |||
| 3 months postintake | .18 | .33 | .17 | .33 | .28 | .41 | .57 | 1.14 | |||
| Monthly seclusions or restraints | 1.97 | 3, 86 | >.1 | ||||||||
| 2 months preintake | .00 | .00 | .21 | .72 | .22 | 1.04 | .17 | .71 | |||
| 3 months postintake | .63 | 2.08 | .06 | .19 | .13 | .39 | .26 | .61 | |||
| Overt Aggression Scale item 2 score (toward objects)a | 2.71 | 3, 85 | .05 | ||||||||
| Study intake | .21 | .51 | 1.13 | 1.48 | .70 | 1.06 | .50 | 1.15 | |||
| 3 months postintake | .80 | 1.38 | .71 | 1.12 | .83 | 1.30 | .50 | .79 | |||
| Overt Aggression Scale item 3 score (assault of others)a | 2.66 | 3, 85 | .053 | ||||||||
| Study intake | .38 | 1.01 | 1.29 | 1.46 | .78 | 1.09 | .72 | 1.23 | |||
| 3 months postintake | 1.00 | 1.80 | .96 | .96 | 1.61 | 1.90 | .72 | 1.18 | |||
| Brief Psychiatric Rating Scale scoreb | 1.61 | 3, 85 | >.1 | ||||||||
| Study intake | 45.58 | 16.03 | 50.46 | 18.55 | 51.26 | 17.41 | 42.67 | 11.81 | |||
| 3 months | 51.6 | 20.24 | 51.13 | 20.65 | 47.09 | 13.25 | 49.22 | 13.69 | |||
| Life Skills Profile–20 scorec | .07 | 3, 85 | >.1 | ||||||||
| Study intake | 39.96 | 7.92 | 47.00 | 8.17 | 41.70 | 9.38 | 41.17 | 10.80 | |||
| 3 months | 39.12 | 9.13 | 45.29 | 9.76 | 40.30 | 11.22 | 40.39 | 12.26 | |||
| Greystone Intrusiveness Measure scored | 1.75 | 3, 77 | >.1 | ||||||||
| Study intake | 2.48 | 1.25 | 2.86 | 1.36 | 3.05 | 1.36 | 2.41 | 1.50 | |||
| 3 months | 2.24 | 1.42 | 2.88 | 1.45 | 2.96 | 1.72 | 2.89 | 1.57 | |||
| Pet Attitude Scale scoree | .63 | 3, 60 | >.1 | ||||||||
| Study intake | 100.88 | 11.19 | 92.23 | 18.20 | 94.53 | 20.51 | 99.31 | 13.33 | |||
| 3 months | 104.60 | 9.76 | 101.42 | 12.07 | 91.30 | 23.29 | 97.48 | 16.68 | |||
Table 2 Three-month outcomes, versus preintake and baseline, among inpatients receiving animal-assisted or social skills psychotherapy or regular hospital care
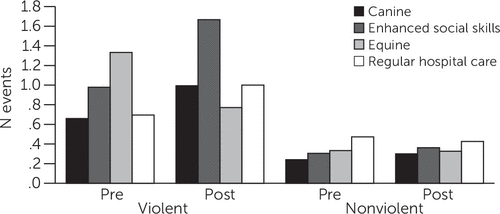
Figure 1 Violent and nonviolent events pre- and postintake among psychiatric inpatientsa
a Mean number of monthly incident reports were for the two months before study intake and the three months after study intake for each of the intervention groups, including canine- or equine-assisted psychotherapy, enhanced social skills psychotherapy (active control), and regular hospital care (standard control).
Secondary aggression-related measures also suggested improvement with AAT (Table 2). The OAS-M showed group differences in aggression against objects and persons (p=.05 and p=.053, respectively). Aggression among EAP participants appeared to decrease, compared with increased or unchanged levels for other groups (Table 2), with post hoc tests suggesting EAP benefits (p=.029 versus CAP for objects; p=.074 versus SSP for persons, respectively). Group differences were also suggested for 1:1 observation, often a consequence of aggressive behavior (p=.051), with post hoc tests suggesting benefits of canine (p=.058) as well as equine (p=.082) therapy versus regular hospital care. No differences were evident for seclusion and restraint (Table 2).
Nonviolent incidents showed no differences pre- versus postintervention (Table 2 and Figure 1). Similarly, no AAT effects were found with the BPRS, LSP-20, GIM (Table 2), or mood measures on the visual analogue scales (data not shown).
Covariance analyses revealed that few factors contributed to AAT effects on violence. Adding recruitment rationale (aggressive versus regressed) to the model did not reduce the AAT effect on violent incidents (F=2.97, df=3 and 85, p=.036), nor were effects found for baseline BPRS, LSP-20, or GIM scores; attitudes about pets; or age, sex, diagnosis, or sessions attended. To determine whether the reduction in EAP-related violent incidents was associated with reduced symptoms (BPRS), improved function (LSP-20), or reduced intrusiveness (GIM), we tested pre-post changes with each of these covariates in separate analyses. Change in BPRS score had no effect on violence, with the intervention group effect retained (F=3.03, df=3 and 84, p=.034); improved LSP-20 score was associated with reduced incidents (F=5.41, df=1 and 84, p=.022), with effect of intervention group retained (F=3.01, df=3 and 84, p=.05). Improved intrusiveness was associated with reduced violence (F=5.62, df=1 and 76, p=.02) and with a diminished group effect (F=1.91, df=3 and 76, ns).
Staff expectations of AAT benefits at baseline, before randomization, were associated with AAT effects on violence (rated on a 4-point scale, for “very helpful,” “somewhat helpful,” “little or no help,” and “may be detrimental”). In the model, staff expectations were associated with reduced violent incidents (F=6.99, df=1 and 82, p=.01), and the AAT group effect was retained (F=3.65, df=3 and 82, p=.016). Table 3 shows that violent incidents appeared unchanged among patients in the EAP group for whom staff had lesser expectations, whereas those for whom staff predicted that AAT would be “very helpful” showed a large decrease in incidents.
| Canine assisted | Equine assisted | Enhanced social skills | Regular hospital care | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staff-predicted result of AATb | N | M | SD | N | M | SD | N | M | SD | N | M | SD |
| May be detrimental | 0 | 0 | 0 | 0 | ||||||||
| Little or no help | 4 | .25 | .88 | 6 | .00 | .7 | 4 | 2.08 | 3.98 | 1 | 3.83 | |
| Somewhat helpful | 14 | .33 | 1.16 | 9 | .07 | .85 | 7 | .60 | 1.42 | 11 | .03 | .45 |
| Very helpful | 6 | .44 | 1.01 | 9 | –1.57 | 2.46 | 10 | .32 | .98 | 6 | .22 | .54 |
Table 3 Change in violent events from intake to 3-month postintake among inpatients receiving animal-assisted or social skills psychotherapy or regular hospital carea
Discussion
Interactions with animals have been employed clinically in many settings, with benefits reported for psychiatric and other medical patients in alleviating affective symptoms and in improving interpersonal interactions (3,5,6). To our knowledge, this is the first controlled study of the effects of equine- and canine-assisted therapy on violent behavior among long-term psychiatric patients. Our findings, using independently reported clinical incident reports as well as staff observations with use of the OAS-M, showed that EAP was associated with reduced violence for at least several months after treatment initiation. Moreover, the need for 1:1 clinical observation appeared reduced by both canine and equine therapies. The failure to otherwise detect effects for canine therapy, an established treatment modality in many clinical settings, including ours, suggests a unique benefit for EAP in reducing violence in this population. Lack of CAP effects, however, may have been related to the relatively low incidence of preintervention violent incidents in the CAP group, making beneficial effects more difficult to detect (Table 2 and Figure 1).
This study should be considered in its specialized clinical context of patients with high levels of psychiatric disability requiring long-term hospitalization in an era of active discharge-oriented treatment. Considering the numerous environmental and treatment factors likely to affect patient behavior during the five months of study, it is noteworthy that a weekly intervention of less than an hour on at most ten occasions (for some, considerably fewer) had a detectable effect on a serious and at times intractable dimension of behavior. Observations for the non-AAT control groups suggest that the period of study was otherwise a challenging one, associated with relatively increased violent events throughout the hospital. EAP appears to have buffered these effects and contributed to reducing violence below preintervention levels.
A two-month baseline was selected so as to detect established behavioral patterns while being sufficiently brief to capture data for a large majority of potential participants. Findings for the three-month outcome interval, which only partly included the period of AAT interventions, suggest that EAP benefits extended for at least several weeks beyond the period of equine contact. Whether benefits persist beyond the treatment interval and succeeding weeks requires study. Further, considering the variable attendance, we found no evidence that the number of sessions attended was predictive of outcomes. The apparent lack of a “dose effect” suggests that fewer than ten EAP sessions may be sufficient. This could increase the feasibility of using EAP even considering its costs, the modest number of patients who can be accommodated per session, and the brief duration of most psychiatric hospitalizations.
Our findings suggest that EAP may be beneficial for a broad range of psychiatric patients with extended hospitalizations. Covariance analyses did not suggest that age, sex, race-ethnicity, chart diagnosis, symptom severity, legal commitment status, attitudes toward animals, or length of hospitalization predicted benefit from EAP. Similarly, the selection criterion (violent versus regressed) made no substantive contribution to outcome, and our clinical impressions were that group processes and interactions for patients initially identified as violent or regressed were quite comparable. Finally, diminished violence in the group receiving EAP appeared to be a specific effect rather than a function of global symptomatic improvement (BPRS scores). It was, not unexpectedly, associated with reduced intrusiveness (GIM scores). Improved clinical function (LSP-20 scores) was associated with reduced violence but did not fully account for the EAP effect.
There have been few controlled studies of AAT for hospitalized psychiatric patients; most of these studies have been limited to patients working with dogs. Canine-assisted interactions have been associated with immediate reductions in anxiety or depression (1,14) and fear of a medical procedure (15), with improved self-esteem and psychiatric symptomatology (over several months) (16), and with improved social functioning but not with impulse control among older patients with schizophrenia (2). Unique effects from therapy horses may come from interacting with physically imposing animals that appear quite capable of causing harm but do not. Equine interactions may model nonviolent behavioral strategies, resulting in patients’ greater tolerance of provocative interpersonal stimuli. Nonpredatory equines, tending to mirror rather than direct human responses, may have a therapeutic advantage for some patients over more predatory species, such as canines and humans (17,18). This may be especially relevant to patients with a history of interpersonal trauma, a focus of future study.
Several considerations emerged as relevant to AAT optimization with chronic psychiatric patients. Adherence to standard therapeutic approaches for each AAT modality was feasible, with minor adjustments (such as briefer sessions) (7). Progress through the EAP protocols, however, was more variable than EAP therapists anticipated. In the study, random assignment to intervention group may have diminished some effects because it precluded matching patients with preferred therapy animals. Indeed, anticipation of interacting with horses dampened the enthusiasm of some patients assigned to other groups, although attendance did not differ significantly. Using patient preferences and the predictive power of staff impressions is likely to enhance the effectiveness of AAT.
Equine-assisted group therapy that follows standard therapeutic approaches, such as recommended by EAGALA, is resource intensive. It requires construction and maintenance of the physical environment, attention to risk reduction, identification and transport of appropriate therapy horses, recruitment of trained equine therapists, integration of AAT sessions (subject to weather-related cancellation) with other ongoing hospital programs, escorting patients to treatment sites, and monitoring within-session safety and clinical needs. Our AAT program benefited from intervention teams that worked together over time. Interactions of AAT therapists (with little prior exposure to highly symptomatic patients) with GPPH clinicians (many with little exposure to horses) appeared essential to developing sustainable treatment models that adhere to established AAT principles.
Conclusions
AAT, and especially EAP, may be an effective therapeutic modality for long-term psychiatric patients at risk of violence. It is uncertain whether the current standardized AAT interventions can be generalized to other animal-assisted interventions, such as unstructured exposure to pets or visiting animals, therapeutic riding, or structured one-on-one AAT. As the first such controlled study of EAP in a complex naturalistic clinical setting, replications using similar and disparate intervention models and populations are essential.
1 : The effects of animal-assisted therapy on anxiety ratings of hospitalized psychiatric patients. Psychiatric Services 49:797–801, 1998Link, Google Scholar
2 : Animal-assisted therapy for elderly schizophrenic patients: a one-year controlled trial. American Journal of Geriatric Psychiatry 9:439–442, 2001Crossref, Medline, Google Scholar
3 : Animal-facilitated therapy in various patient populations: systematic literature review. Holistic Nursing Practice 24:187–203, 2010Crossref, Medline, Google Scholar
4 : Animal assisted interventions in mental health: definitions and theoretical foundations; in Handbook on Animal Assisted Therapy, 3rd ed. Edited by Fine AH. Burlington, Mass, Academic Press, 2010Crossref, Google Scholar
5 : Animal-assisted therapy: a meta-analysis. Anthrozoos 20:225–238, 2007Crossref, Google Scholar
6 : Handbook on Animal Assisted Therapy, 3rd ed. Burlington, Mass, Academic Press, 2010Google Scholar
7 EAGALA: What is the EAGALA Model? Santaquin, Utah, EAGALA. Available at www.eagala.org/Information/What_Is_EAGALA_ModelGoogle Scholar
8 : Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. Journal of Neuropsychiatry and Clinical Neurosciences 3:S44–S51, 1991Medline, Google Scholar
9 : The Brief Psychiatric Rating Scale (BPRS). Psychological Reports 10:799–812, 1962Crossref, Google Scholar
10 : Development of a brief form of the Life Skills Profile: the LSP-20. Australian and New Zealand Journal of Psychiatry 35:677–683, 2001Crossref, Medline, Google Scholar
11 : Assessing intimidation using a brief intrusiveness measure. Clinical Schizophrenia and Related Psychoses 1:193–195, 2007Crossref, Google Scholar
12 : Modification of the Pet Attitude Scale. Society and Animals 12:137–142, 2004Crossref, Google Scholar
13 : The use of visual analog scales in mood disorders: a critical review. Journal of Psychiatric Research 31:569–579, 1997Crossref, Medline, Google Scholar
14 : Animal-assisted therapy: effects on stress, mood, and pain. Journal of Lancaster General Hospital 6:56–59, 2011Google Scholar
15 : Effects of animal-assisted therapy on patients’ anxiety, fear, and depression before ECT. Journal of ECT 19:38–44, 2003Crossref, Medline, Google Scholar
16 : The effect of animal-assisted activity on inpatients with schizophrenia. Journal of Psychosocial Nursing and Mental Health Services 47:42–48, 2009Crossref, Medline, Google Scholar
17 : Guest editorial: equine-assisted therapy. Journal of Rehabilitation Research and Development 48:ix–xii, 2011Crossref, Medline, Google Scholar
18 : Equine-facilitated psychotherapy with adult female survivors of abuse. Journal of Psychosocial Nursing 46:36–42, 2008Google Scholar



