Cost-Effectiveness of Collaborative Care for Depression in a Primary Care Veteran Population
Abstract
OBJECTIVE: This study examined the incremental cost-effectiveness of a collaborative care intervention for depression compared with consult-liaison care. METHODS: A total of 354 patients in a Department of Veterans Affairs (VA) primary care clinic who met the criteria for major depression or dysthymia were randomly assigned to one of the two care models. Under the collaborative care model, a mental health team provided a treatment plan to primary care providers, telephoned patients to encourage adherence, reviewed treatment results, and suggested modifications. Outcomes were assessed at three and nine months by telephone interviews. Health care use and costs were also assessed. RESULTS: A significantly greater number of collaborative care patients were treated for depression and given prescriptions for antidepressants. The collaborative care patients experienced an average of 14.6 additional depression-free days over the nine months. The mean incremental cost of the intervention per patient was $237 for depression treatment and $519 for total outpatient costs. A majority of the additional expenditures were accounted for by the intervention. The incremental cost-effectiveness ratio was $24 per depression-free day for depression treatment costs and $33 for total outpatient cost. CONCLUSIONS: Better coordination and communication under collaborative care was associated with a greater number of patients being treated for depression and with moderate increases in days free of depression and in treatment cost. Additional resources are needed for effective collaborative care models for depression treatment in primary care.
Most depression treatment takes place in primary care (1,2), where the condition continues to be underdetected and undertreated (3,4,5,6,7). Symptoms of depression are associated with elevated health care costs in both general and veteran populations (8,9,10,11,12).
Collaborative care is based on a chronic illness model and incorporates components at the patient, provider, and system level (13). It is a population-based approach in which multidisciplinary teams assist primary care providers in delivering evidence-based treatment (14,15). Studies have shown that such interventions are successful in improving both the process and the outcomes of depression care (16,17,18,19,20).
We adapted a collaborative care model to the patient population and treatment resources in a Department of Veterans Affairs (VA) primary care setting and compared it with a traditional treatment model—consult-liaison care. In previous studies, this collaborative care model was associated with more rapid improvement in depression symptoms and in mental health status compared with consult-liaison care (21,22).
Information on the cost-effectiveness of treatment can assist administrators and policy makers to balance the costs and outcomes of treatments. Studies have shown that organized depression treatment programs are associated with higher treatment costs due to additional visits and antidepressant prescriptions (23,24,25,26,27). The studies of collaborative care interventions show that the increase in annual depression treatment costs was in the range of $264 to $487 for the intervention group (23). Such studies also found no cost-offset effects—that is, no significant reductions in total medical care costs for the intervention group compared with traditional care (23,24,25,26,27,28).
Previous interventions were conducted in general populations, primarily among middle-aged women (2,16,18,19,20,29). The VA system serves a primarily aging male population in a lower socioeconomic bracket and with a much greater prevalence of chronic illness and comorbid psychiatric illness (30,31,32). However, it has not been clear whether the cost of collaborative care would be higher for this population. In the study reported here we sought to determine the impact of a collaborative care intervention for depression on the use and costs of care. We also assessed the cost-effectiveness of collaborative care as well as incremental depression treatment costs associated with the intervention.
Methods
The study was conducted in the general internal medicine clinic (GIMC) of the Seattle VA Medical Center. The GIMC is organized into four groups of providers to which providers and their patient panels are assigned in an unsystematic manner (33). At the start of the study period—January 1998 to March 1999—GIMC staff included 19 attending physicians, 38 residents, ten fellows, and 22 nurse practitioners. The clinic was supported by one full-time-equivalent (FTE) psychiatry resident, one clinical psychologist, one clinical psychology intern, four social workers, and two social work interns. Two of the four provider groups were randomly assigned to the collaborative care intervention and two to consult-liaison care.
We used several methods to locate eligible patients, including referral from two ongoing, unrelated studies (34,35); a prevention survey conducted at the clinic; and referral by primary care providers. After initial screening, each prospective participant was given a computer-assisted structured interview to assess the severity of depression, current and past use of medication or therapy, health status, current and past alcohol use, symptoms of posttraumatic stress disorder (PTSD), history of mental illness, and barriers to care. Assessment of depression and anxiety symptoms was based on the PRIME-MD (36), with additional questions taken from the Structured Clinical Interview for DSM-IV (4). The interview was administered by an experienced psychology technician in person or by telephone.
Of 1,125 patients who were screened, 732 completed the assessment interview. Of these, 500 who had a current major depressive episode, dysthymia, or both were eligible to participate in the study. We limited the exclusion criteria to the extent possible in order to maximize the generalizability of the results and included patients receiving ongoing intensive treatment for depression, patients requiring treatment for substance abuse or PTSD before initiating depression treatment, and patients with acute suicidality, psychosis, or another condition requiring immediate treatment. The study enrolled 354 patients who consented to participate, 168 of whom were assigned to the collaborative care group and 186 to the consult-liaison care group. All procedures were approved by the human subjects committee of the University of Washington.
Clinical outcomes were assessed through telephone interviews three and nine months after enrollment. Follow-up assessments were completed for 146 patients (87 percent) in the collaborative care group and 164 (88 percent) in the consult-liaison group. There were no significant differences in baseline characteristics between respondents and nonrespondents. These interviews included repeat administration of a 20-item depression scale extracted from the Symptom Checklist-90 (SCL-90) (37)—the primary outcome measure—and other measures not presented here.
The collaborative care program was a multifaceted intervention that included diagnosis and treatment, patient education, and patient support and progress evaluation. The collaborative care team consisted of a clinical psychologist, a psychiatrist, a social worker, and a psychology technician. The team met weekly to develop treatment plans and conduct six- and 12-week progress evaluations. Treatment plans were developed on the basis of the VA's major depression guideline, taking into account current and previous treatment and patients' preferences. The team communicated with primary care providers by using electronic progress notes and tracked receipt and acknowledgement of notes and follow-up. If the agreed-upon prescriptions were not written in a timely fashion, the study team contacted the provider to discuss the recommendation.
Depression treatment options were to begin, increase the dosage of, or change the antidepressant medication; add an adjuvant medication; enroll in a cognitive-behavioral therapy group; schedule an appointment with the psychologist or the psychiatrist; or refer the patient to mental health specialty care (38,39). Options were selected in a stepwise fashion beginning with the least resource-intensive option. If later evaluations indicated that the option was not effective, a new or stepped-up option was recommended. No limits on specialty care visits were imposed.
The patient education component of the intervention included a videotape (40) and a workbook, which were mailed to each patient. To evaluate the patients' progress and provide support to the patients, a social work staff member or student telephoned each patient on a regular basis to encourage adherence, address treatment barriers, and assess response to the intervention.
Consult-liaison care represented the traditional model in which the primary care provider was responsible for initiating treatment with consultation from or referral to specialist care as needed. To ensure that this approach was not merely a test of more versus less care, an explicit attempt was made to equate the amount of mental health resources available to each provider group, including the available time of the psychologist and social workers. Psychiatry residents' time was reserved for the consult-liaison provider groups. The supervising psychiatrist participated in the collaborative care intervention but rarely met directly with patients. All providers were notified of the diagnosis. Providers of consult-liaison care were able to refer patients to the psychiatry resident, psychologists, or social workers who were physically present in the GIMC. Patients with more complicated conditions were referred to specialty mental health clinics, facilitated by the study team. For both types of care, the primary care providers received three hours of instruction about assessment and treatment of depression (41).
On the basis of the approach developed by Lave and colleagues (24) and modified by Simon and colleagues (25), SCL depression scores from baseline and follow-up assessments were used to calculate the number of depression-free days during the nine-month follow-up period. This method uses depression severity data from two consecutive outcome assessments to estimate the severity of depression for each day during the interval by linear interpolation. Days for which the SCL depression score was .5 or less were considered depression free. Days for which the SCL depression score was 2.0 or greater were considered fully symptomatic. Days with intermediate severity scores were assigned a value between depression free and fully symptomatic by linear interpolation—for example, a day with an SCL score of 1.25 would be considered 50 percent depression free. Calculation of depression-free days was limited to participants who completed all follow-up assessments.
Data on the use of VA care in the nine-month follow-up period were obtained from the local VA data warehouse, including outpatient visits, inpatient admissions, and outpatient prescriptions filled. We categorized visits as primary care visits, mental health specialty visits, or other treatment visits on the basis of clinic identifiers. Mental health specialty visits included visits to mental health, PTSD, or substance abuse clinics. A primary care depression visit was defined as a primary care visit with an ICD-9 code of 296.2, 296.3, 298.0, 300.4, 309.1, or 311, regardless of primary or secondary diagnosis.
Costs of care for the nine-month study period were estimated on the basis of national average cost estimates from the Cost Distribution Report (CDR), the VA's cost accounting system (42). The CDR provides an average cost per visit in a specific clinic. The cost estimates included direct and indirect costs and were adjusted to year 2000 dollars by using the medical component of the consumer price index. The intervention costs that were not reflected in the utilization data—that is, team meetings and follow-up patient telephone calls—were estimated by sampling staff activity records and computing average cost on the basis of event duration by using actual input costs, such as labor, fringe benefits, and overhead costs. On average, team meetings lasted 56 minutes, involved discussion of 13 patients, and cost $403. Three follow-up calls were scheduled during the acute phase of treatment and five during the maintenance phase. Completed follow-up calls took 24 minutes—including preparation, unsuccessful attempts to call, and documentation—and cost $15 on average.
Our primary cost analysis was depression treatment cost, which included estimated costs during the nine-month period for all primary care depression visits, outpatient mental health specialty visits, antidepressant prescriptions, and the intervention program (for patients receiving collaborative care). In a secondary analysis, we considered total outpatient costs and total costs (inpatient and outpatient). However, the sample was not sufficiently large to provide statistical power to detect even moderate differences in total costs (43).
Confidence intervals (CIs) for depression-free days and cost measures were estimated by bootstrapping with 1,000 replications (44,45,46). Adjusted differences between collaborative care and consult-liaison care were estimated by using regression models with bootstrap interval estimates. All analyses were adjusted for baseline characteristics, including age, gender, race, marital status, living situation (whether the patient was living alone), previous depression, SCL depression score, Chronic Disease Score (47,48), and the logarithm of cost in the previous year. All models were also adjusted for interclustering correlation at the provider level by using Huber's estimator from a robust regression (49,50). All cost and utilization comparisons included the full study sample. The cost-effectiveness analysis included patients who completed all follow-up assessments.
Results
Baseline characteristics of the study participants are summarized in Table 1. No significant differences between groups were noted in baseline demographic characteristics, SCL depression scores, or costs during the year before enrollment, except that the patients receiving collaborative care were more likely to have had previous depressive episodes than patients receiving consult-liaison care (56 percent compared with 48 percent, p<.05). The number of depression-free days in the nine-month follow-up period was calculated as the areas under the curves—that is, the proportion of depression-free days multiplied by follow-up time interval. By this measure, the mean±SD number of depression-free days was 112.7± 81.1 for the collaborative care group and 107.3±75.6 for the consult-liaison care group in the nine-month period. The proportion of depression free days in the collaborative care and consult-liaison groups, respectively, was 37 percent versus 36 percent at baseline, 43 percent versus 36 percent at three months, and 42 percent versus 41 percent at nine months. After baseline characteristics were adjusted for, the difference between the two groups was 14.6 days (p=.059, 95 percent CI=−.5 to 29.6).
Use of VA aftercare in the nine-month follow-up period is shown in Table 2. Compared with patients receiving consult-liaison care, those receiving collaborative care were significantly more likely to have a primary care depression visit (84 percent compared with 56 percent) and to be given a prescription for an antidepressant (80 percent compared with 61 percent). No significant differences were noted in the number of primary care visits, use of primary care for nondepression care, or use of mental health specialty services.
The utilization pattern reflects the study interventions. Patients in the collaborative care group were more likely to visit a primary care provider for depression treatment than those in the consult-liaison group (77 percent compared with 39), with significantly more visits per patient (2.57±3.82 compared with 1.63± 4.61). Twenty-four percent of the patients in the collaborative care group received cognitive-behavioral therapy. By intervention design, the patients who received collaborative care were less likely to be referred to GIMC psychiatrists than those in consult-liaison care (3 percent compared with 35 percent), with significantly fewer mean visits per patient (.05±.37 compared with 1.80±2.50).
Table 3 shows the cost of care per patient for the nine-month follow-up period. The costs of depression treatment were approximately $160 higher for the collaborative care group, primarily because of the cost of intervention program ($182). The estimated cost for mental health specialty visits was greater for the consult-liaison care group than for the collaborative care group ($174 compared with $86). Greater differences between the two groups were found in broader categories of costs (a $615 difference in outpatient costs and a $1,257 difference in total cost). CIs increased as the cost category broadened, reducing the precision of the latter cost estimates.
Adjusted incremental cost and cost-effectiveness of the intervention are shown in Table 4. On average, the adjusted incremental cost of collaborative care was $237 for depression treatment costs (CI=$70 to $404) and $519 for total outpatient costs (CI=$47 to $519). For total cost, the adjusted incremental cost was considerably smaller ($169). For total outpatient cost and total cost, we could not conclude a finding of cost offset (negative incremental cost).
When only depression treatment costs were considered, the additional cost per depression-free day was approximately $24. On the basis of cost-effectiveness ratios for depression treatment costs, total outpatient costs, and total cost, we could not conclude a finding of absolute cost savings (negative incremental cost per depression-free day), because their CIs included zero. The wide CIs reflect the uncertainty in estimates of both incremental effectiveness and incremental cost.
Our final analysis examined the likelihood that either collaborative care or consult-liaison care would demonstrate both greater effectiveness and lower cost—in the jargon of cost-effectiveness, that one of the approaches would be "dominant." For depression treatment costs, consult-liaison care was found to have both greater effectiveness and lower cost only 34 times in 1,000 bootstrap replications, indicating a 3.4 percent probability that consult-liaison care would be dominant over collaborative care. We did not observe dominance of the collaborative care intervention in 1,000 replications. The results show a 96.6 percent probability that the collaborative care intervention would be associated with both increased cost and increased effectiveness.
Discussion and conclusions
We found that a collaborative care model designed to improve depression treatment in a veteran primary care population was associated with modest increases in time free of depression and in treatment costs over the nine-month study period. However, the difference in the number of depression-free days between groups was not statistically significant. This result was similar to the findings for a collaborative care model for depression relapse prevention for chronically depressed patients, who were more comparable in illness severity to our study patients (28). The relapse-prevention program demonstrated 13.9 additional depression-free days (not significant), with an incremental cost of $273 for outpatient depression treatment cost in a 12-month period.
Our results are consistent with those of other depression treatment interventions in primary care, which suggests that additional resources are needed to achieve better outcomes for patients with major depression (23,25,26,27,28). We did not find any cost-offset effect—that is, improved depression treatment was not associated with lower total outpatient care costs (23,25,26,27,28). Instead, we estimated an average increment of $519 in total outpatient costs for collaborative care.
This intervention was developed on the basis of the availability of treatment resources in VA primary care clinics, including team treatment meetings, brief follow-up telephone calls, and cognitive-behavioral therapy. This intervention was less resource-intensive than Katon's models, which required either additional psychiatrist or psychologist visits during the treatment period (16,18). Furthermore, Katon's studies were conducted in a managed care network with a considerably different patient population. The patients in our study had a number of characteristics that predict difficulties with treatment, such as older age, male gender, less education, a higher rate of unemployment, and more chronic illness and comorbid psychiatric illness. Our findings demonstrate that even in a population that is very difficult to treat, systematically reorganizing the delivery of mental health services by using a collaborative care model enables more patients with depression to be treated in primary care with a modest incremental cost.
This evaluation was not intended to assess the cost-effectiveness of collaborative care compared with a control group of patients receiving no treatment for depression. The consult-liaison care provided to the patients in this study involved a fairly high level of mental health services. Thus our results imply that better communication and coordination between mental health and primary care providers can increase the number of patients treated, even in a primary care setting already providing a broad range of mental health services. Collaborative care may have larger effects when introduced in a system that has fewer mental health resources already in place.
The relatively low cost of telephone follow-up ($15 per call and $49 per patient) was due to the use of adjunctive clinical personnel to conduct this task. In the early stage of the intervention, the calls were made by GIMC social workers. Because of lack of system resources as the social workers' caseload and range of duties increased over time, the telephone calls were later made by students supervised by social workers. Experience with this study and other studies suggest that staff performing this care management function need telephone assessment and triage skills but do not need to be psychotherapists or prescribers (31,51). Our experience also implies that a successful adoption of population-based treatment care management through telephone follow-up will require identification of the appropriate clinic staff to perform this function and integration of this activity with other duties.
This study had several limitations. First, our results, based on a single site with existing mental health integration in primary care, may not be generalizable to other primary care settings. Second, this study did not include utilization and cost data for services obtained outside the VA. Third, the VA cost accounting method provides an average cost per clinic visit, which does not capture variation in outpatient cost across visits with different intensities of care. Finally, our calculation of depression-fee days was based on the SCL depression scale rather than the Hamilton Depression Scale used in previous studies.
Comparing these findings with those for other interventions requires use of a common measure of effectiveness, such as cost per quality-adjusted life year (QALY). The literature suggests that depression reduces the value of a life-year by .2 to .4 QALYs (52,53,54,55,56,57). On the basis of this range of conversion, our estimate of $23.50 per additional depression-free day would be equal to an estimated $21,444 to $42,838 per additional QALY for depression treatment costs, which was comparable to Katon's stepped collaborative care and relapse-prevention models (25,28). These results, consistent with those of other studies, compare favorably with those for a wide range of preventive and therapeutic services (58,59).
Acknowledgments
This research was supported by grant II-R-95-097 from the Health Services Research and Development Service of the Department of Veterans Affairs. The authors thank Jan Buchanan, M.S.W., M.C., Rocco Bagala, A.C.S.W., Diane Greenberg, Ph.D., and Grady Paden, M.D., for their participation in study implementation.
Dr. Liu, Dr. Hedrick, and Dr. Fihn are affiliated with the Health Services Research and Development Center of Excellence at the VA Puget Sound Health Care System, with which Ms. Hasenberg was affiliated at the time of this study, and with the department of health services of the University of Washington in Seattle. Dr. Chaney and Dr. Felker are with the mental health service of the VA Puget Sound Health Care System and the department of psychiatry and behavioral sciences of the University of Washington. Dr. Heagerty is with the department of biostatistics and Dr. Katon is with the department of psychiatry and behavioral sciences at the university. Address correspondence to Dr. Liu, HSRD (152), VA Puget Sound Health Care System, 1660 South Columbian Way, Seattle, Washington 98108 (e-mail, [email protected]). An earlier version of this paper was presented at the annual meeting of the VA Health Services Research and Development Service held February 14-16, 2001, in Washington, D.C.
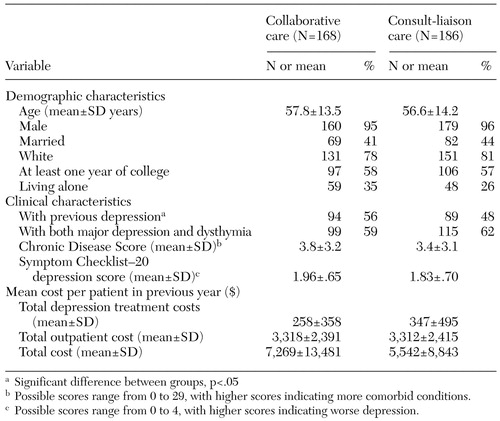 |
Table 1. Baseline characteristics of veterans receiving either collaborative care or consult-liaison care for depression
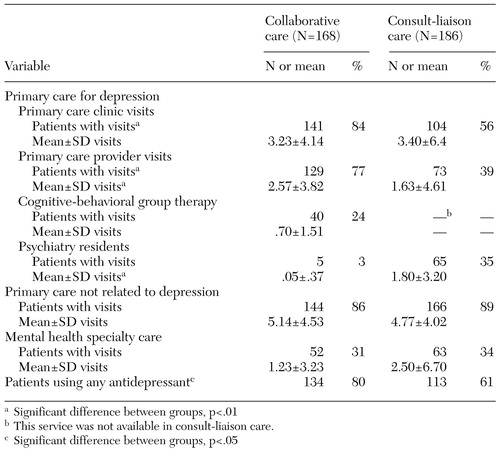 |
Table 2. Unadjusted service use among veterans receiving either collaborative care or consult-liaison care for depression during the nine months after baseline
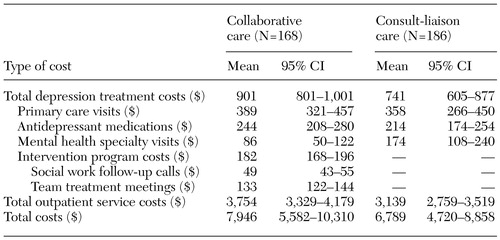 |
Table 3. Unadjusted cost of care per patient for nine months after baseline in a sample of veterans receiving either collaborative care or consult-liaison care for depression
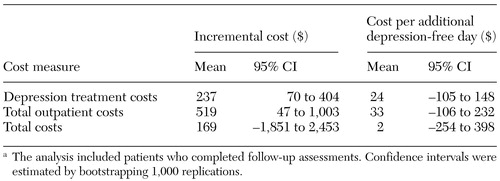 |
Table 4. Adjusted incremental cost and cost-effectiveness of a collaborative care intervention for veterans with depressiona
a The analysis included patients who completed follow-up assessments. Confidence intervals were estimated by bootstrapping 1,000 replications.
1. Linn LS, Yager J: The effect of screening, sensitization, and feedback on notation of depression. Journal of Medical Education 55:942-949, 1980Medline, Google Scholar
2. Wells KB, Sherbourne C, Schoenbaum M, et al: Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. JAMA 283:212-220, 2000Crossref, Medline, Google Scholar
3. Magruder HK, Zung WW, Feussner JR, et al: Management of general medical patients with symptoms of depression. General Hospital Psychiatry 11:201-206, 1989Crossref, Medline, Google Scholar
4. Spitzer RL, Williams JB, Gibbon M, et al: The Structured Clinical Interview for DSM-III-R (SCID): history, rationale, and description. Archives of General Psychiatry 49:624-629, 1992Crossref, Medline, Google Scholar
5. Coyne JC, Schwenk TL, Fechner BS: Nondetection of depression by primary care physicians reconsidered. General Hospital Psychiatry 17:3-12, 1995Crossref, Medline, Google Scholar
6. Lyness JM, Cox C, Curry J, et al: Older age and the underreporting of depressive symptoms. Journal of the American Geriatrics Society 43:216-221, 1995Crossref, Medline, Google Scholar
7. Coyne JC, Schwenk TL: AHCPR depression guidelines: countering misconceptions with more misconceptions? American Psychologist 50:452-453, 1995Google Scholar
8. Manning WG Jr, Wells KB: The effects of psychological distress and psychological well-being on use of medical services. Medical Care 30:541-53, 1992Crossref, Medline, Google Scholar
9. Johnson JG, Spitzer RL, Williams JB, et al: Psychiatric comorbidity, health status, and functional impairment associated with alcohol abuse and dependence in primary care patients: findings of the PRIME MD-1000 study. Journal of Consulting and Clinical Psychology 63:133-140, 1995Crossref, Medline, Google Scholar
10. Simon G, Ormel J, VonKorff M, et al: Health care costs associated with depressive and anxiety disorders in primary care. American Journal of Psychiatry 152:352-357, 1995Link, Google Scholar
11. Simon GE, VonKorff M, Barlow W: Health care costs of primary care patients with recognized depression. Archives of General Psychiatry 52:850-856, 1995Crossref, Medline, Google Scholar
12. Druss BG, Rohrbaugh RM, Rosenheck RA: Depressive symptoms and health costs in older medical patients. American Journal of Psychiatry 156:477-479, 1999Abstract, Google Scholar
13. Antonuccio D: Psychotherapy for depression: no stronger medicine. American Psychologist 50:450-452, 1995Crossref, Medline, Google Scholar
14. Hays RD, Wells KB, Sherbourne CD, et al: Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Archives of General Psychiatry 52:11-19, 1995Crossref, Medline, Google Scholar
15. Ganzini L, Lee MA, Heintz RT, et al: The effect of depression treatment on elderly patients' preferences for life-sustaining medical therapy. American Journal of Psychiatry 151:1631-1636, 1994Link, Google Scholar
16. Katon W, Von-Korff M, Lin E, et al: Collaborative management to achieve treatment guidelines: impact on depression in primary care. JAMA 273:1026-1031, 1995Crossref, Medline, Google Scholar
17. Katon W, Rutter C, Ludman EJ, et al: A randomized trial of relapse prevention of depression in primary care. Archives of General Psychiatry 58:241-247, 2001Crossref, Medline, Google Scholar
18. Katon W, Robinson P, Von-Korff M, et al: A multifaceted intervention to improve treatment of depression in primary care. Archives of General Psychiatry 53:924-932, 1996Crossref, Medline, Google Scholar
19. Katon W, Von Korff M, Lin E, et al: Stepped collaborative care for primary care patients with persistent symptoms of depression. Archives of General Psychiatry 56:1109-1115, 1999Crossref, Medline, Google Scholar
20. Katzelnick DJ, Simon GE, Pearson SD, et al: Randomized trial of a depression management program in high utilizers of medical care. Archives of Family Medicine 9:345-351, 2000Crossref, Medline, Google Scholar
21. Hedrick SC, Chaney EF, Liu CF, et al: Process of Care in Innovative and Traditional Treatments for Depression in VA Primary Care: Reallocating Resources. Presented at the annual meeting of the Department of Veterans Affairs Health Services Research and Development Service, Washington, DC, Feb 14-165, 2001Google Scholar
22. Hedrick SC, Chaney EF, Felker B, et al: Effectiveness of collaborative care depression treatment in Veterans' Affairs primary care. Journal of General Internal Medicine 18:9-16, 2003Crossref, Medline, Google Scholar
23. Von Korff M, Katon W, Bush T, et al: Treatment costs, cost offset, and cost-effectiveness of collaborative management of depression. Psychosomatic Medicine 60:143-149, 1998Crossref, Medline, Google Scholar
24. Lave JR, Frank RG, Schulberg HC, et al: Cost-effectiveness of treatments for major depression in primary care practice. Archives of General Psychiatry 55:645-651, 1998Crossref, Medline, Google Scholar
25. Simon GE, Katon WJ, VonKorff M, et al: Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. American Journal of Psychiatry 158:1638-1644, 2001Link, Google Scholar
26. Simon GE, Manning WG, Katzelnick DJ, et al: Cost-effectiveness of systematic depression treatment for high utilizers of general medical care. Archives of General Psychiatry 58:181-187, 2001Crossref, Medline, Google Scholar
27. Schoenbaum M, Unutzer J, Sherbourne C, et al: Cost-effectiveness of practice-initiated quality improvement for depression: results of a randomized controlled trial. JAMA 286:1325-1330, 2001Crossref, Medline, Google Scholar
28. Simon GE, Von Korff M, Ludman EJ, et al: Cost-effectiveness of a program to prevent depression relapse in primary care. Medical Care 40:941-950, 2002Crossref, Medline, Google Scholar
29. Schulberg HC, Block MR, Madonia MJ, et al: Treating major depression in primary care practice: eight-month clinical outcomes. Archives of General Psychiatry 53:913-919, 1996Crossref, Medline, Google Scholar
30. Randall M, Kilpatrick KE, Pendergast JF, et al: Differences in patient characteristics between Veterans Administration and community hospitals: implications for VA planning. Medical Care 25:1099-1104, 1987Crossref, Medline, Google Scholar
31. Brody DS, Khaliq AA, Thompson TL: Patients' perspectives on the management of emotional distress in primary care settings. Journal of General Internal Medicine 12:403-406, 1997Crossref, Medline, Google Scholar
32. Kazis LE, Ren XS, Lee A, et al: Health status in VA patients: results from the Veterans Health Study. American Journal of Medical Quality 14:28-38, 1999Crossref, Medline, Google Scholar
33. Cebul RD: Randomized, controlled trials using the metro firm system. Medical Care 29:JS9-JS18, 1991Google Scholar
34. McDonnell M, Anderson S, Fihn S: The Ambulatory Care Quality Improvement Project: A Multi-Site Information System for Monitoring Health Outcomes. Presented at the annual meeting of the Department of Veterans Affairs Health Services Research and Development Service, Washington, DC, Feb 11-13, 1998Google Scholar
35. Williams JW Jr, Barrett J, Oxman T, et al: Treatment of dysthymia and minor depression in primary care: a randomized controlled trial in older adults. JAMA 284:1519-1526, 2000Crossref, Medline, Google Scholar
36. Spitzer RL, Williams JBW, Kroenke K, et al: Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 Study. JAMA 272:1749-1756, 1994Crossref, Medline, Google Scholar
37. Derogatis LR, Lipman RS, Rickels R, et al: The Hopkins Symptom Checklist: a measure of primary symptom dimensions. Psychological Measurements in Psychopharmacology 7:79-110, 1974Google Scholar
38. Clinical Practice Guideline: Depression in Primary Care, vol 2, Treatment of Major Depression. Rockville, Md, Agency for Health Care Policy and Research, 1993Google Scholar
39. Schulberg HC, Katon W, Simon GE, et al: Treating major depression in primary care practice: an update of the Agency for Health Care Policy and Research practice guidelines. Archives of General Psychiatry 55:1121-1127, 1998Crossref, Medline, Google Scholar
40. Depression (Recurrent and Chronic) [videotape]. New York, Time-Life Medical Patient Educational Media, 1996Google Scholar
41. Major Depressive Disorder Clinical Guidelines. Washington, DC, Department of Veterans Affairs, Under Secretary for Health, 1997Google Scholar
42. Barnett PG: Review of methods to determine VA health care costs. Medical Care 37:AS9-AS17, 1999Google Scholar
43. Sturm R, Unutzer J, Katon W: Effectiveness research and implications for study design: sample size and statistical power. General Hospital Psychiatry 21:274-283, 1999Crossref, Medline, Google Scholar
44. Manning W, Frytak D, Weinstein M: Reflecting uncertainty in cost-effectiveness analyses, in Cost-Effectiveness in Health and Medicine. Edited by Gold M, Siegel J, Russel L, et al. New York, Oxford University Press, 1996Google Scholar
45. Briggs AH, Wonderling DE, Mooney CZ: Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Economics 6:327-340, 1997Crossref, Medline, Google Scholar
46. Chaudhary MA, Stearns SC: Estimating confidence intervals for cost-effectiveness ratios: an example from a randomized trial. Statistics in Medicine 15:1447-1458, 1996Crossref, Medline, Google Scholar
47. VonKorff M, Wagner EH, Saunders K: A chronic disease score from automated pharmacy data. Journal of Clinical Epidemiology 45:197-203, 1992Crossref, Medline, Google Scholar
48. Clark DO, VonKorff M, Saunders K, et al: A chronic disease score with empirically derived weights. Medical Care 33:783-795, 1995Crossref, Medline, Google Scholar
49. Huber PJ: The behavior of maximum likelihood estimates under nonstandard conditions, in Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, University of California Press, 1967Google Scholar
50. STATA 7 Reference Manual. College Station, Tex, Stata Corp, 2001Google Scholar
51. Simon GE, Von Korff M, Rutter C, et al: Randomized trial of monitoring feedback and management of care by telephone to improve treatment of depression in primary care. British Medical Journal 320:550-554, 2000Crossref, Medline, Google Scholar
52. Wells KB, Sherbourne CD: Functioning and utility for current health of patients with depression or chronic medical conditions in managed, primary care practices. Archives of General Psychiatry 56:897-904, 1999Crossref, Medline, Google Scholar
53. Unutzer J, Patrick D, Diehr P, et al: Quality adjusted life years in older adults with depressive symptoms and chronic medical conditions. International Psychogeriatrics 15-33, 2000Google Scholar
54. Revicki DA, Wood M: Patient-assigned health state utilities for depression-related outcomes: differences by depression severity and antidepressant medications. Journal of Affective Disorders 48:25-36, 1998Crossref, Medline, Google Scholar
55. Kaplan R: Health-related quality of life in mental health services evaluation, in Cost-Effectiveness of Psychotherapy: A Guide for Practitioners, Researchers, and Policy-Makers. Edited by Miller N, Magruder K. New York, Oxford University Press, 1999Google Scholar
56. Fryback DG, Dasbach EJ, Klein R, et al: The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Medical Decision Making 13:89-102, 1993Crossref, Medline, Google Scholar
57. Pyne JM, Patterson TL, Kaplan RM, et al: Preliminary longitudinal assessment of quality of life in patients with major depression. Psychopharmacology Bulletin 33:23-29, 1997Medline, Google Scholar
58. Gold M, Siegel J, Russell L, et al: Cost-Effectiveness in Health and Medicine. New York, Oxford University Press, 1996Google Scholar
59. Tengs TO, Adams ME, Pliskin JS, et al: Five-hundred life-saving interventions and their cost-effectiveness. Risk Analysis 15:369-90, 1995Crossref, Medline, Google Scholar



