Special Section on Implications of CATIE: What CATIE Found: Results From the Schizophrenia Trial
Although studies of second-generation antipsychotics in schizophrenia have indicated that these drugs are comparable to the first-generation antipsychotics in reducing psychotic symptoms and that they produce few neurologic effects, with the exception of clozapine, the evidence for their superior efficacy and safety has been inconsistent ( 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 ). As a result of dominant prescribing preferences for second-generation antipsychotics over first-generation antipsychotics, despite their greater cost, questions have been raised about the clinical advantages and the cost-effectiveness of the second-generation antipsychotics.
This article provides an overview of the primary outcomes of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), which was supported by the National Institute of Mental Health and designed to compare the effectiveness of first- and second-generation antipsychotic medications ( 11 , 12 , 13 , 14 ). In this overview we review the design, methods, and results of CATIE, with a focus on the implications and limitations of the trial.
CATIE design and procedures
Design and measures
The rationale, design, and methods of CATIE have been previously described ( 11 , 12 , 13 , 14 ). The study was conducted between October 2001 and December 2004 at 57 U.S. clinical sites. Patients were randomly assigned to receive olanzapine, perphenazine, quetiapine, risperidone, or ziprasidone under double-blind conditions and were followed for up to 18 months or until treatment was discontinued for any reason (phase 1) ( Table 1 ). Patients with tardive dyskinesia (N=231, or 15% of the sample) were excluded from being randomly assigned to receive perphenazine and were assigned to one of the four second-generation antipsychotics (phase 1A). Ziprasidone was added to the trial after 40% of the patients had been enrolled. The initial randomization thus had four separate strata within which patients had an equal chance of being randomly assigned to the treatments: before ziprasidone was introduced to the study, patients with tardive dyskinesia would be randomly assigned equally to receive olanzapine, quetiapine, or risperidone; before ziprasidone was introduced to the study, patients without tardive dyskinesia would be randomly assigned equally to receive olanzapine, quetiapine, risperidone, or perphenazine; and after ziprasidone was added to the study, these same two groups would be randomly assigned equally to receive the same antipsychotics plus ziprasidone. If the phase 1 treatment was perphenazine, patients who discontinued perphenazine were then randomly assigned to double-blinded treatment with olanzapine, quetiapine, or risperidone (phase 1B). If patients in phase 1B again discontinued treatment, then they entered phase 2.
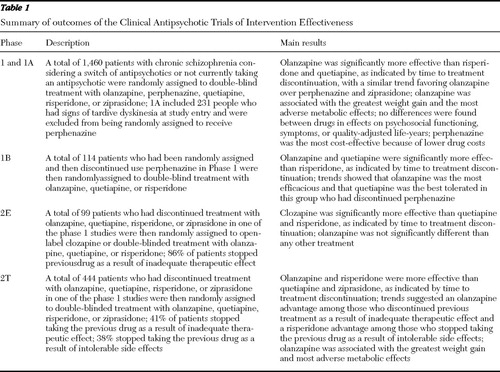 |
In phase 2, patients and their study doctor could choose between two randomization pathways. The "efficacy" pathway (phase 2E), recommended to individuals who discontinued the previous treatment as a result of inefficacy, compared open-label clozapine to double-blinded treatment with olanzapine, quetiapine, or risperidone. The "tolerability" pathway (phase 2T), recommended to individuals who discontinued the previous treatment as a result of intolerability, compared double-blinded treatment with olanzapine, quetiapine, risperidone, or ziprasidone.
Participants
Eligible patients were 18 to 65 years of age, had received a research diagnosis of schizophrenia, and were found to be able to take oral antipsychotic medication. Patients were excluded if they had a diagnosis of schizoaffective disorder, mental retardation, or other cognitive disorders; had a history of serious adverse reactions to the proposed treatments; had had only one schizophrenic episode; had a history of treatment resistance; were pregnant or were breast-feeding; or had a serious and unstable medical condition.
The study was approved by the institutional review board at each site, and written informed consent was obtained from the patients or their legal guardians.
Interventions
Identical-appearing capsules contained olanzapine (7.5 mg), quetiapine (200 mg), risperidone (1.5 mg), or perphenazine (8 mg). After January 2002 ziprasidone (40 mg) was added. The dosage of the medications was flexible, ranging from one to four capsules daily. Concomitant medications were permitted throughout the trial, except for additional antipsychotic agents. Patients had monthly study visits with the study physician and other research staff for clinical and research assessments, unless additional visits were needed.
General analysis approach
A consistent statistical plan for treatment group comparisons was generally applied across the studies involving CATIE data ( 13 ), although the approach to cost-effectiveness analyses ( 15 ) was somewhat different because of the focus on multiple phases of treatment. Analyses for phase 1 were conducted on four data sets with overlapping membership on the basis of the tardive dyskinesia and ziprasidone cohort stratification. Each data set included only patients with an equal chance of being randomly assigned to the treatments under comparison. Patients assigned to the perphenazine group, in particular, were compared only with patients who did not have tardive dyskinesia, and patients assigned to the ziprasidone group were compared with only other patients who were randomly assigned to groups after ziprasidone was added. The primary comparison was an overall test between the four treatments available at the beginning of the trial, excluding patients with tardive dyskinesia and those randomly assigned to the ziprasidone group. If the overall test was significant at p<.05, then pairwise treatment group comparisons were conducted by using step-down testing and a Hochberg adjustment for multiple comparisons ( 16 ). Ziprasidone was separately compared with the other four drugs among patients randomly assigned to groups after ziprasidone became available, but patients with tardive dyskinesia were excluded from the perphenazine comparison.
The outcome measure of time until treatment discontinuation was evaluated by using survival analysis methods. Continuous outcome measures were evaluated at specific monthly intervals by using analysis of covariance (ANCOVA) and adjusting for relevant baseline characteristics at end of phase (by using ANCOVA with additional adjustment for a patient's duration in phase 1) and across all visits by using repeated-measures mixed models. Side effect event rates were evaluated by using Poisson regression, which accounted for a patient's duration in phase 1. Analyses in the studies using phase 2 data followed similar methods.
Published reports of CATIE findings
Initial phase 1 report
This initial phase 1 report evaluated the relative effectiveness of second-generation antipsychotics as compared with a first-generation antipsychotic, perphenazine ( 17 ). Only a minority of patients in each group remained on the assigned drug treatment for the duration of this study (discontinuation rates ranged from 64% to 82%).
Seventy-four percent of patients discontinued the study medication before 18 months (olanzapine, 64%; perphenazine, 75%; quetiapine, 82%; risperidone, 74%; and ziprasidone, 79%). The time to treatment discontinuation for all causes was longest with olanzapine, compared with quetiapine (p<.001) and risperidone (p<.002). The comparisons of olanzapine with perphenazine (p<.021) and with ziprasidone (p<.028) produced similar results, but they did not meet the researchers' a priori standard of statistical significance, which adjusted for multiple comparisons. Times to discontinuation because of intolerable side effects or extrapyramidal effects for the treatments were not significantly different. Patients assigned to olanzapine had the longest time to discontinuation as a result of inadequate efficacy, the longest successful treatment time, and the fewest hospitalizations as a result of exacerbation of schizophrenia ( Table 1 ). However, substantial increases in weight were more common with olanzapine, as were the largest adverse effects on hemoglobin A1C, total cholesterol, and triglycerides.
Neurocognitive outcomes
Keefe and colleagues ( 18 ) compared the neurocognitive effects in phase 1 among 817 patients who completed longitudinal neurocognitive assessments. The primary aim was to delineate differences in the neurocognitive effects of these five treatments at the two-month assessment period. Neurocognition was slightly but significantly improved from baseline at each of the time points for all treatment groups. At two months, treatment resulted in small neurocognitive improvements of z=.12 for olanzapine (p<.003), .23 for perphenazine (p<.001), .17 for quetiapine (p<.001), and .24 for risperidone (p<.001), with no significant differences between treatment groups. For those who completed 18 months of treatment on the initially assigned medication, neurocognitive improvement was greater in the perphenazine group than in the olanzapine group (p=.001) and in the risperidone group (p=.038) but not in the quetiapine group. The ziprasidone group did not differ from any of the other treatment groups. Neurocognitive improvement predicted longer time to treatment discontinuation, independently from symptom improvement, among patients treated with quetiapine or ziprasidone.
Keefe and colleagues ( 18 ) concluded that all of the antipsychotic treatment groups had a small but significant improvement in neurocognition; however, there was no difference between the groups after two months of treatment, and the improvements in neurocognition were not likely to be greater than placebo or practice effects.
Psychosocial functioning
Swartz and colleagues ( 19 ) evaluated whether improvement in psychosocial functioning was significantly different between phase 1 and phase 2 treatment groups. Psychosocial functioning was assessed by using the Quality of Life Scale (QLS). Although modest improvements were evident across treatment groups, there were no significant differences between treatment groups and there was no distinct superiority of any antipsychotic in improving psychosocial functioning. Magnitude of improvement from baseline, based on within-group comparisons, was largely comparable at 12 months (average effect size .19 standard deviation units) for the olanzapine (.19), risperidone (.26), perphenazine (.19) and ziprasidone (.26) groups; improvement was somewhat less for quetiapine (.09), although gains for the quetiapine treatment group were more comparable with those in the other drug groups at six and 18 months.
Results were similar at six and 18 months, and there were no significant differences between the treatment groups in the amount of change in the QLS total score or subscales at six, 12, or 18 months. Patients treated with clozapine in the efficacy pathway made comparable gains. Early treatment discontinuations, especially among patients most impaired at baseline, limited the ability to achieve more substantial functional gains. More substantial improvements would likely require more intensive adjunctive psychosocial rehabilitation interventions.
Staying versus switching
Essock and colleagues ( 20 ) examined the impact of "staying versus switching" in phase 1 of the trial. Among patients who were randomly assigned to the olanzapine group or the risperidone group, those who were taking the same antipsychotic upon study entry had significantly longer times until discontinuation than did those who were taking different antipsychotics upon study entry. When these "stayers" were removed from the analyses, the original pattern of phase 1 results remained, although differences seen in the original analyses were attenuated. Essock and colleagues concluded that comparisons of medication effectiveness should take into account whether each of the medications being compared were newly started.
Cost-effectiveness
Rosenheck and colleagues ( 15 ) reported on the cost-effectiveness analyses of the trial evaluated from the perspective of each medication as a treatment initiation strategy and including any subsequent antipsychotic medications used in phase 2 or 3 as part of that patient's treatment trajectory. As a result these intention-to-treat analyses included all available observations, classified by initial drug assignment, and the costs of reassignment of most patients to another second-generation drug. The cost analysis included medications plus health services use. Quality-adjusted life years (QALYs) were assessed on the basis of Positive and Negative Syndrome Scale (PANSS) subscales ( 21 ) and side effect rating scales.
Although there was modest improvement over 18 months for all groups as measured by QALYs, there were no significant differences between perphenazine and any second-generation medication. Average total monthly health care costs were $300–$500 (20%–30%) lower for perphenazine than for second-generation antipsychotics, because of lower antipsychotic drug costs for perphenazine. There were no significant differences between treatments on QALYS, on the PANSS itself, or on three other measures of quality of life, and there were no differences when observations after phase 1 were excluded.
Rosenheck and colleagues ( 15 ) concluded that initiating treatment with perphenazine was less costly and no less effective than initiating treatment with each of the four second-generation antipsychotics as measured by the PANSS and by a disease-specific measure of QALYs that combined measures of symptoms and side effects.
Phase 1B outcomes
Stroup and colleagues ( 22 ) compared the effectiveness of the second-generation antipsychotics olanzapine, quetiapine, and risperidone among patients who had just discontinued the older antipsychotic perphenazine. The time to treatment discontinuation was longer for patients treated with quetiapine (median 9.9 months) and olanzapine (7.1 months) than with risperidone (3.6 months). There were no significant differences between treatments in the subgroups that discontinued as a result of inefficacy, intolerability, or patient decision. Stroup and colleagues concluded that in a group of patients with chronic schizophrenia who had just discontinued the older antipsychotic perphenazine, the antipsychotics quetiapine and olanzapine were more effective than risperidone, as reflected by longer time to discontinuation for any reason. They concluded that the effectiveness and acceptability of antipsychotic drugs appear to vary considerably according to clinical circumstances.
Phase 2E outcomes
In the efficacy arm of phase 2, McEvoy and colleagues ( 23 ) compared the effectiveness of switching to clozapine versus another second-generation antipsychotic among patients who had discontinued treatment with a second-generation antipsychotic as a result of inefficacy in phase 1 of the trial. Ninety-nine patients who had just discontinued treatment with olanzapine, quetiapine, risperidone, or ziprasidone were randomly assigned to treatment with clozapine or another, newer second-generation antipsychotic not previously received in the trial. Time to all-cause treatment discontinuation was significantly longer for clozapine (median 10.5 months) than for quetiapine (median 3.3 months) or risperidone (median 2.8 months) but not for olanzapine (median 2.7 months). Time to discontinuation for inadequate therapeutic effect was significantly longer for clozapine than for olanzapine, quetiapine, or risperidone. At three-month assessments, PANSS total scores had decreased significantly more among patients treated with clozapine (-11.7) than among patients treated with quetiapine (-2.5) or risperidone (-4.1), although all decreases were modest. (Possible scores on the PANSS range from 30 to 210, with higher scores indicating greater symptom severity.)
The authors concluded that for patients with schizophrenia with inadequate response to a second-generation antipsychotic, clozapine was more effective than switching to another newer second-generation antipsychotic, although clozapine's advantage was compromised by weight gain and metabolic sequelae.
Phase 2T outcomes
Stroup and colleagues ( 24 ) evaluated the primary outcome of time to all-cause discontinuation from the tolerability arm of phase 2. In phase 2T, 444 patients who discontinued phase 1 as a result of intolerability were randomly assigned to receive a drug that they had not previously received in the study—that is, olanzapine, quetiapine, risperidone, or ziprasidone. Although the intent of this phase was to evaluate patients who discontinued phase 1 as a result of intolerability, because there was a choice of which arm of phase 2 to enter, some patients and their doctors who had stopped the previous treatment as a result of inadequate efficacy chose this arm, presumably to avoid possible random assignment to clozapine.
The time to treatment discontinuation was longer for patients treated with risperidone (median 7.0 months) and olanzapine (median 6.3 months) than with quetiapine (median 4.0 months) and ziprasidone (median 2.8 months). Among the patients who discontinued the previous treatment as a result of inefficacy, olanzapine was more effective than quetiapine and ziprasidone, and risperidone was more effective than quetiapine. There were no significant differences between treatments among those who discontinued the previous treatment as a result of intolerability.
The authors concluded that for patients who had just discontinued a second-generation antipsychotic medication, risperidone and olanzapine were more effective than quetiapine and ziprasidone, as reflected by longer time to all-cause discontinuation.
Implications of CATIE
The finding in CATIE that perphenazine was not significantly different in overall effectiveness compared with olanzapine, quetiapine, risperidone, and ziprasidone and the finding that perphenazine was the most cost-effective drug was a surprise to many observers. Although the CATIE results are controversial, the results of an earlier Department of Veterans Affairs Cooperative study ( 25 ), a recent study of second-generation antipsychotics from the United Kingdom ( 26 ), and a meta-analysis of side-effects with low-potency antipsychotic drugs ( 4 ) are all broadly consistent with the results of CATIE, demonstrating no consistent overall benefits of second-generation antipsychotics.
The CATIE results also suggested that clinical circumstances affected drug effectiveness and that these circumstances may be useful in making treatment choices. Clozapine was confirmed as being the most effective drug for individuals with a poor symptom response to previous antipsychotic drug trials, although clozapine is encumbered by troublesome adverse effects. Olanzapine was consistently favorable in overall effectiveness and efficacy, but it was also consistently associated with adverse metabolic risks. Risperidone was consistently effective overall, combining good efficacy and tolerability. However, it was not as effective as clozapine for individuals who had stopped previous medications as a result of inefficacy, and it was not as effective as quetiapine among individuals who had just discontinued the first-generation drug perphenazine. Quetiapine, although similar in some ways to olanzapine in adverse effects, seemed to be a good choice for people who had discontinued perphenazine, and it was particularly well tolerated in this group, presumably because of substantial differences in drug action. Ziprasidone was consistently weight neutral and had the best profile of metabolic effects of all drugs studied in phases 1 and 2 of CATIE, although it was not generally efficacious in the dosage employed in the trial.
Several concerns about the design and implementation of the trial ( 27 , 28 , 29 ) are discussed below.
Tardive dyskinesia risk
The CATIE finding of no difference in tardive dyskinesia risk across treatment groups is of concern, because a lowered risk of tardive dyskinesia may remain an important advantage of second-generation antipsychotics and the study may have had limited power to detect new cases of tardive dyskinesia. For example, the older and chronic nature of the sample may have reduced the incidence of new cases of tardive dyskinesia. A recent comprehensive review of past studies on tardive dyskinesia by Correll and colleagues ( 30 ) reported a 4.6% lower annual risk of tardive dyskinesia with second-generation antipsychotics, compared with first-generation antipsychotics, although the authors noted that the results could have been biased by the fact that all three head-to-head first- to second-generation comparison trials involved moderate to high doses of haloperidol. The CATIE finding of no difference in tardive dyskinesia risk across treatment groups is consistent with the findings of a meta-analysis of 31 randomized controlled trials, which found no greater risk of extrapyramidal symptoms between low-potency first-generation antipsychotics and second-generation antipsychotics other than clozapine ( 1 ). However, a finding of "no difference" could also be due to an inadequate design to detect new tardive dyskinesia cases or low statistical power. However, it is still possible that the advantages of second-generation antipsychotics in regard to extrapyramidal symptoms and tardive dyskinesia have been overstated or conflated with secular prescribing trends toward more moderate dosing of all antipsychotics.
Exclusion of tardive dyskinesia from the perphenazine group
The initial reason for excluding patients with tardive dyskinesia from random assignment to perphenazine was the belief in 2000 by senior scientific experts who provided input to the National Institute of Mental Health that patients with tardive dyskinesia should not be exposed to any first-generation antipsychotic. Within the non-tardive dyskinesia group (85% of the sample), patients had an equal chance of being assigned to each of the five available treatments.
It is argued that the exclusion of patients with tardive dyskinesia from assignment to perphenazine may have biased results by preventing perphenazine from being given to patients with more severe systems or patients who were treatment resistant. However, patients who had tardive dyskinesia at baseline were not involved in any between-group comparisons involving perphenazine and second-generation antipsychotics. All perphenazine comparisons were limited to the non-tardive dyskinesia randomization strata, allowing unbiased comparisons. However, because the study weakly generalizes to patients at higher risk of tardive dyskinesia and because of the profound effects of perceived tardive dyskinesia risk on prescribing, some critics have suggested that the cost-effectiveness and other findings should be interpreted with caution ( 27 ).
Choice of dosing
Concern has been expressed that the mean modal dose of olanzapine was higher than in typical practice at the time of the CATIE study and that dosing of risperidone and ziprasidone were somewhat lower than in typical practice (28,29). Although drugs that were dosed higher than optimal might have had an advantage in terms of measures of effectiveness, those dosed lower would have a presumptive advantage in terms of side effects. Thus, any dose-related biases favoring effectiveness could have been offset by disadvantages in side effects. Although the possibility that other dosing regimens might have led to different conclusions cannot be ruled out, this uncertainty is no greater in CATIE than in any of the other flexibly dosed double-blind trials of second-generation antipsychotics.
Other concerns
The analysis by Essock and co-authors ( 20 ) regarding staying on an antipsychotic medication recently prescribed at baseline versus switching raises the specter that staying on one's pre-study drug may have offered an unfair advantage to more popular drugs, such as olanzapine and risperidone. However, that study suggests that the same superiority trends persist even after controlling for baseline drug regimen.
Conclusions
CATIE results have provoked strong reactions and extensive discussions among researchers, clinicians, consumers, and policy makers. Because CATIE was large and independently funded, the results, in particular the "unexpectedly" good effectiveness and cost-effectiveness of perphenazine, have gotten a great deal of attention. Putting the results in context however, CATIE results are in fact quite consistent with other independently funded studies ( 25 , 26 ), meta-analyses ( 1 , 2 , 3 ), and other systematic reviews ( 10 ).
Acknowledgments and disclosures
CATIE was supported by grant N01-MH-90001 from the National Institute of Mental Health (NIMH). A list of study locations and principal investigators can be found at www.catie.unc.edu/schizophrenia/locations.html. This article was written as part of Dr. Hsiao's official duties as a government employee. The views expressed in this article do not necessarily represent the views of NIMH, the National Institutes of Health, the Department of Health and Human Services, or the U.S. government.
Dr. Swartz has been a consultant for, speaker for, or has received research funding from Eli Lilly and Company, Pfizer, and Bristol-Myers Squibb. Dr. McEvoy has received honoraria from Eli Lilly and Company and Pfizer. Dr. Rosenheck has received research support from Eli Lilly and Company, Janssen Pharmaceutica, Astra-Zeneca, and Wyeth. He has been a consultant to GlaxoSmithKline, Bristol-Myers Squibb, Organon, and Janssen Pharmaceutica. Dr. Keefe is a consultant for, has served on an advisory board for, is a speaker for, has received unrestricted educational funding from, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, Acadia Pharmaceuticals, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Cephalon Pharmaceutical, Cortex Pharmaceuticals, Cyberonics, Dainippon Sumitomo Pharma, Eli Lilly and Company, Gabriel Pharmaceuticals, Johnson & Johnson, Lundbeck Pharmaceuticals, Memory Pharmaceuticals, Merck, Orexigen Therapeutics, Organon Pharmaceuticals, Otsuka America Pharmaceutical, Pfizer, Saegis Pharmaceuticals, Sanofi-Aventis Pharmaceuticals, and XenoPort. Dr. Lieberman has received grants from, received support for research, served as a consultant for, or served on an advisory board for Acadia, Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck, Merck, Organon Pharmaceuticals, Pfizer, and RepliGen Corporation. The other authors report no competing interests.
1. Leucht S, Wahlbeck K, Hamann J, et al: New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet 361:1581–1589, 2003Google Scholar
2. Geddes J, Freemantle N, Harrison P, et al: Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. British Medical Journal 321:1371–1376, 2000Google Scholar
3. Davis JM, Chen N, Glick ID: A meta-analysis of the efficacy of second-generation antipsychotics. Archives of General Psychiatry 60:553–564, 2003Google Scholar
4. Leucht S, Pitschel-Walz G, Abraham D, et al: Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophrenia Research 35:51–68, 1999Google Scholar
5. Leucht S, Barnes TRE, Kissling W, et al: Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. American Journal of Psychiatry 160:1209–1222, 2003Google Scholar
6. Wahlbeck K, Cheine M, Essali A, et al: Evidence of clozapine's effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. American Journal of Psychiatry 156:990–999, 1999Google Scholar
7. Chakos M, Lieberman J, Hoffman E, et al: Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. American Journal of Psychiatry 158:518–526, 2001Google Scholar
8. Tuunainen A, Wahlbeck K, Gilbody S: Newer atypical antipsychotic medication in comparison to clozapine: a systematic review of randomized trials. Schizophrenia Research 56:1–10, 2002Google Scholar
9. Miyamoto S, Duncan GE, Marx CE, et al: Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Molecular Psychiatry 10:79–104, 2005Google Scholar
10. Lehman AF, Kreyenbuhl J, Buchanan RW, et al: The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations. Schizophrenia Bulletin 30:193–217, 2004Google Scholar
11. Stroup TS, McEvoy JP, Swartz MS, et al: The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophrenia Bulletin 29:15–31, 2003Google Scholar
12. Swartz MS, Perkins DO, Stroup TS, et al: Assessing clinical and functional outcomes in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial. Schizophrenia Bulletin 29:33–43, 2003Google Scholar
13. Davis SM, Koch GG, Davis CE, et al: Statistical approaches to effectiveness measurement and outcome-driven re-randomizations in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) studies. Schizophrenia Bulletin 29:73–80, 2003Google Scholar
14. Keefe RS, Mohs RC, Bilder RM, et al: Neurocognitive assessment in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project schizophrenia trial: development, methodology, and rationale. Schizophrenia Bulletin 29:45–55, 2003Google Scholar
15. Rosenheck R, Leslie D, Sindelar J, et al: Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. American Journal of Psychiatry 163:2080–2089, 2006Google Scholar
16. Hochberg Y: A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802, 1988Google Scholar
17. Lieberman JA, Stroup TS, McEvoy JP, et al: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209–1223, 2005Google Scholar
18. Keefe RSE, Bilder RM, Harvey PD, et al: Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia. Archives of General Psychiatry 64:633–647, 2007Google Scholar
19. Swartz MS, Perkins DO, Stroup TS, et al: Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE Study. American Journal of Psychiatry 164:428–436, 2007Google Scholar
20. Essock SM, Covell NH, Davis SM, et al: Effectiveness of switching antipsychotic medications. American Journal of Psychiatry 163:2090–2095, 2006Google Scholar
21. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 13: 261–276, 1987Google Scholar
22. Stroup TS, Lieberman JA, McEvoy JP, et al: Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia after discontinuation of a previous atypical antipsychotic. American Journal of Psychiatry 163:611–622, 2006Google Scholar
23. McEvoy JP, Lieberman JA, Stroup TS, et al: Effectiveness of clozapine, quetiapine and risperidone in patients with chronic schizophrenia who failed prior atypical antipsychotic treatment. American Journal of Psychiatry 153:600–610, 2006Google Scholar
24. Stroup TS, Lieberman JA, McEvoy JP, et al: Effectiveness of olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia after discontinuing perphenazine: a CATIE study. American Journal of Psychiatry 164:415–427, 2007Google Scholar
25. Rosenheck R, Perlick D, Bingham S, et al: Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. JAMA 290:2693–2702, 2003Google Scholar
26. Jones PB, Barnes TRE, Davies L, et al: Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Archives of General Psychiatry 63: 1079–1087, 2006Google Scholar
27. Freedman R, Carpenter WT, Davis JM, et al: The cost of drugs for schizophrenia. American Journal of Psychiatry 163: 2029–2031, 2006Google Scholar
28. Meltzer HY, Bobo WV: Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrum 11 (suppl 7):14–24, 2006Google Scholar
29. Kane JM: Commentary on the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). Journal of Clinical Psychiatry 76:831–832, 2006Google Scholar
30. Correll CU, Leucht S, Kane JM: Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. American Journal of Psychiatry 161:414–425, 2004Google Scholar



